Abstract
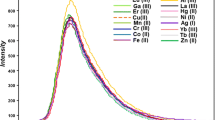
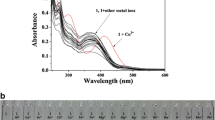
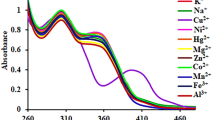
A fluorescent chemosensor including dual Bodipy units (d-BODIPY) was improved for selective copper (II) sensing in half-aqueous samples. The sensor d-BODIPY has a highly selective and sensitive detection towards Cu (II) over the studied competing for metal cations. The interaction among solutions of Cu (II) and d-BODIPY caused a crucial quenching effect in fluorescence maxima at 548 nm (λex = 470 nm) owing to the electronic trap occurring between the amide and triazole units. The quenching effect without any change in wavelength can be explained by a photoinduced electron transfer (PET) process. The binding constant (Ka) of d-BODIPY with Cu (II) was calculated and also the limit of detection of d-BODIPY for Cu (II) was 1.2 × 10−8 M. In addition, the bio-imaging in the yeast cells suggested that d-BODIPY had an excellent potential to be used to investigate Cu (II).








Similar content being viewed by others
References
Türkmen M, Budur D (2018) Heavy metal contaminations in edible wild mushroom species from Turkey’s Black Sea region. Food Chem 254:256–259
Lee J, Park KY, Cho J, Kim JY (2018) Releasing characteristics and fate of heavy metals from phytoremediation crop residues during anaerobic digestion. Chemosphere 191:520–526
Kim J-J, Kim Y-S, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elements in Med Bio 54:226–231
Wang S, Ma L, Liu G, Shouzhi P (2019) Diarylethene-based fluorescent and colorimetric chemosensor for the selective detection of Al3+ and CN−. Dyes Pigments 164:257–266
Normaya E, Fazli M, Ahmad MN, Bulat KHK (2019) COSMO-RS and DFT studies on development and optimization of quercetin as a chemosensor for Fe3+ recognition in aqueous medium. J Mol Struct 1184:538–545
Şenkuytu E, Eçik ET, Çoşut B (2018) Bodipy decorated triazine chemosensors for Ag+ ions with high selectivity and sensitivity. J Luminescence 203:639–645
Koonrugsa N, Fuangswasdi S (2019) Metal ion chemosensors based on diaza-18-crown-6 coupling with azobenzene dye. Spectrochim. Acta Part A: Mol. Biomol. Spect. 215:15–23
Xue Z, Liu T, Liu H (2019) Naked-eye chromogenic and fluorogenic chemosensor for mercury (II) ion based on substituted distyryl BODIPY complex. Dyes Pigments 165:65–70
Raju V, Kumar RS, Tharakeswar Y, Kumar SKA (2019) A multifunctional Schiff-base as chromogenic chemosensor for Mn2+ and fluorescent chemosensor for Zn2+ in semi-aqueous environment. Inorg Chim Acta 493:49–56
Çimen A, Bilgiç A, Yılmaz İ (2015) Chemical modification of silica gel with hydrazine carbothioamide derivative for sorption studies of cu (II), Ni (II) and co (II) ions. Desalination Water Treat. 55:420–430
Çimen A, Bilgiç A, Kursunlu AN, Gübbük İH, Uçan Hİ (2014) Adsorptive removal of co (II), Ni (II), and cu (II) ions from aqueous media using chemically modified sporopollenin of Lycopodium clavatum as novel biosorbent. Desalination Water Treatment 52:4837–4847
Çimen A, Torun M, Bilgiç A (2015) Immobilization of 4-amino-2-hydroxyacetophenone onto silica gel surface and sorption studies of cu (II), Ni (II), and co (II) ions. Desalination Water Treat 53:2106–2116
Feng S, Gao Q, Gao X, Yin J, Jiao Y (2019) Fluorescent sensor for copper(II) ions based on coumarin derivative and its application in cell imaging. Inorg Chem Comm 102:51–56
Sinan B (2019) A simple rhodanine-based fluorescent sensor for mercury and copper: the recognition of Hg2+ in aqueous solution, and Hg2+/Cu2+ in organic solvent. J Photochem Photobio A: Chem 372:235–244
Chandra R, Ghorai A, Patra GK (2018) A simple benzildihydrazone derived colorimetric and fluorescent ‘on–off-on’ sensor for sequential detection of copper(II) and cyanide ions in aqueous solution. Sensors Actuat. B: Chem. 255:701–711
Liu H, Cui S, Shi F, Feng SPS, Gao Q, Gao X, Yin J, Jiao Y (2019) A diarylethene based multi-functional sensor for fluorescent detection of Cd2+ and colorimetric detection of Cu2+. Dyes Pigments 161:34–43
Liu S, Wang Y-M, Han J (2017) Fluorescent chemosensors for copper(II) ion: structure, mechanism and application. J Photochem Photobio C: Photochem Reviews 32:78–103
Gao L, Lü F, Xia H, Ding L, Fang Y (2011) Fluorescent film sensor for copper ion based on an assembled monolayer of pyrene moieties. Spectrochim Acta Part A: Mol Biomol Spect 79:437–442
Liang J, Liu H-B, Wang J (2019) Pyrene-based ratiometric and fluorescent sensor for selective Al3+ detection. Inorg Chim Acta 489:61–66
Liu M, Wei J, Wang Y, Ouyang H, Fu Z (2019) Dopamine-functionalized upconversion nanoparticles as fluorescent sensors for organophosphorus pesticide analysis. Talanta 195:706–712
Shen Y, Zhang X, Zhang Y, Li H, Chen Y (2018) An ICT-modulated strategy to construct colorimetric and ratiometric fluorescent sensor for mitochondria-targeted fluoride ion in cell living. Sensors Actuat. B: Chem. 258:544–549
Hong M-M, Liu A-F, Xu Y, Xu D-M (2016) Synthesis and properties of three novel rhodamine-based fluorescent sensors for Hg2+, Chinese Chem. Lett. 27:989–992
Liu C, Xiao T, Wang Y, Wang F, Chen X (2017) Rhodamine based turn-on fluorescent sensor for Hg2+ and its application of microfluidic system and bioimaging. Tetrahedron 73:5189–5193
Kursunlu AN, Guler E, Ucan HI, Boyle RW (2012) A novel Bodipy-Dipyrrin fluorescent probe: synthesis and recognition behaviour towards Fe (II) and Zn (II). Dyes Pigments 94:496–502
Taner B, Kursunlu AN, Güler E (2014) The example of calix[4]pyrrole derivative containing Bodipy unit: Fluorometric and colorimetric sensor for F− ion. Spectrochim. Acta Part A: Mol. Biomol. Spect. 118:903–907
Li H, Sun X, Zheng T, Xu Z, Song Y, Gua X (2019) Coumarin-based multifunctional chemosensor for arginine/lysine and Cu2+/Al3+ ions and its Cu2+ complex as colorimetric and fluorescent sensor for biothiols. Sensors Actuat B: Chem 279:400–409
Wanichacheva N, Kumsorn P, Sangsuwan R, Kamkaew A, Lee VS, Grudpan K (2011) A new fluorescent sensor bearing three dansyl fluorophores for highly sensitive and selective detection of mercury(II) ions. Tetrahedron Lett 52:6133–6136
Quan L, Sun T, Lin W, Guan X, Zheng M, Xie Z, Jing X (2014) BODIPY fluorescent Chemosensor for Cu2+ detection and its applications in living cells: fast response and high sensitivity. J Fluorescence 24:841–846
Abeywardana S, Cantu AL, Nedic T, Schramm MP (2018) Preparation of diverse BODIPY diesters. Tetrahedron Lett 59:3393–3396
Jiang X, Zhang T, Sun C, Meng Y, Xiao L (2019) Synthesis of aza-BODIPY dyes bearing the naphthyl groups at 1,7-positions and application for singlet oxygen generation, Chinese Chem. Lett. 30:1055–1058
Kursunlu AN, Deveci P, Guler E (2013) Synthesis and spectroscopic–electrochemical properties of novel ratiometric hg (II) chemosensor containing Bodipy and the N-phenylaza-15-crown-5 moiety. J Lumin 136:430–436
Yildiz EA, Sevinc G, Yaglioglu HG, Hayvali M (2019) Strategies towards enhancing the efficiency of BODIPY dyes in dye sensitized solar cells. J. Photochem. Photobio. A: Chem. 375:148–157
Shimizu K, Kitagawa D, Kobatake S (2019) Solid emission color tuning of polymers consisting of BODIPY and styrene in various ratios. Dyes Pigments 161:341–346
He S-J, Xie Y-W, Chen Q-Y (2018) A NIR-BODIPY derivative for sensing copper (II) in blood and mitochondrial imaging. Spectrochim. Acta Part A: Mol. Biomol. Spect. 195:210–214
Chen Y, Zhao L, Jiang J (2017) The naphthoate-modifying Cu2+ detective Bodipy sensors with the fluorescent ON-OFF performance unaffected by molecular configuration Spectrochim Acta part a: Mol. Biomol Spect 175:269–275
Chen Y, Pan H, Wang F, Zhao Y, Yin H, Chen Y, Zhang J, Jiang J (2019) An ultrafast BODIPY single molecular sensor for multi-analytes (acid/base/Cu2+/Bi3+) with different sensing mechanism. Dyes Pigments 165:279–286
Tümay SO, Okutan E, Sengul IF, Özcan E, Kandemir H, Doruk T, Çetin M, Çoşut B (2016) Naked-eye fluorescent sensor for cu(II) based on indole conjugate BODIPY dye. Polyhedron 117:161–171
Acknowledgments
The authors gratefully thanks to Tübitak (118Z039) for financial support of this work. The authors express their appreciation to Emine Bagci for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kursunlu, A.N., Ozmen, M. & Güler, E. A Novel Fluorescent Chemosensor for cu (II) Ion: Click Synthesis of Dual-Bodipy Including the Triazole Groups and Bioimaging of Yeast Cells. J Fluoresc 29, 1321–1329 (2019). https://doi.org/10.1007/s10895-019-02456-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02456-3