Abstract
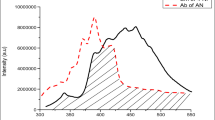
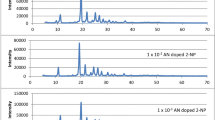
The number of anthracene-tetracene (AN-TN) doped p-terphenyl (p-TP) luminophors [(TN-AN/ p-TP) (D-A)] and thin films of polystyrene doped p-TP luminophors were prepared at different proportion by conventional technique called solid state reaction and spin coating technique respectively. Excitation energy transfer (EET) was studied by fluorimetry and cyclic voltammetry technique. The result showed that, TN-AN/ p-TP in crystalline state as well as in thin films exhibit outstanding green emission at 475–550 nm, peaking at 525 nm. Structural properties and thermal stability were studied by XRD, SEM and TGA-DSC. The HOMO and LUMO energy levels obtained by CV were in the range from 5.82–5.85 eV and 2.94–2.97 eV, respectively. The Eg calculated from the CV found 2.88 eV which are in close agreement with efficient energy transfer in prepared organoluminophors.







Similar content being viewed by others
References
Hosokawa C, Eida M, Matsuura M, Fukuoka K, Nakamura H, Kusumoto T (1997) Organic multi-color electroluminescence display with fine pixels. Synth Met 91:3–7
Forrest SR, Burrows PE, Shen Z, Gu G, Bulovic V, Thompson ME (1997) The stacked OLED (SOLEd): a new type of organic device for achieving high resolution full-color displays. Synth Met 91:9–13
Tullo AH (2001) Competition looms between LCDs and new organic light-emitting diode technology. Chem Eng News 79:49–54
Fuhrmann T, Salbeck J (2003) Organic materials for photonic devices. MRS Bull 28:354–359
Hung LS, Chen CH (2002) Recent progress of molecular organic electroluminescent matrials and devices. Mater Sci Eng R 39:143–222
Yao YS, Zhou QX, Wang XS, Wang Y, Zhang BW (2006) Fine tuning of the photophysical and electroluminescent properties of DCM-type dyes by changing the structure of the electron-donating group. J Mater Chem 16:3512
Jung BJ, Lee JI, Chu HY, Lee LM, Shim HK (2005) A new family of bis-DCM based dopants for red OLEDs. J Mater Chem 15:2470–2475
Tong Q-X, Lai S-L, Chan M-Y, Zhou Y-C, Kwong H-L, Lee C-S (2008) High efficiency nondoped green organic light emitting devices. Chem Phys Lett 455:79–82
Okumoto K, Kanno H, Hamaa Y, Takahashi H, Shibata K (2006) Green fluorescent organic light-emitting device with external quantum efficiency of nearly 10%. Appl Phys Lett 89:063504
Bhalla V, Vij V, Tejpal R, Singh G, Kumar M (2013) Solvent dependent competition between fluorescence resonance energy transfer and through bond energy transfer in rhodamine appended hexaphenylbenzene derivatives for sensing of Hg2+ ions. Dalton Trans 42:4456–4463
Yu JW, Kim JK, Kim DY, Kim C, Song NW, Kim D (2006) Prediction of efficient energy transfer in emissive polymer blends based on Forster radius and the excited state lifetime of acceptors. Curr Appl Phys 6(6):59–65
Lian H, Dai Y, Yang D, Cheng Z, Li C, Hou Z, Shang M, Lin J (2014) Morphology control, luminescence and energy transfer properties of NaCeF4and NaCeF4:Tb3+/Yb3+nanocrystals. Nanoscale 6:9703–9712
Gawrys P, Marszalek T, Bartnik E, Kucinska M, Ulanski J, Zagorska M (2011) Novel, low-cost, highly soluble n-type semiconductors: Tetraazaanthracene Tetraesters. Org Lett 13:6090–6093
Emslie AG, Bonner FT, Peck LG (1958) Flow of a viscous liquid on a rotating disk. J Appl Phys 29:858–862
Desai NK, Kolekar GB, Patil SR (2012) Preparation and characterization of anthracene doped p_terphenyl polycrystalline powders for scintillation application. International Journal of Luminescence and its application 2(I):38–40
Wang X, Lau KC, Li WK (2011) Doping Effects on Structural and Electronic Properties of Ladderanes and Ladder Polysilanes: A Density Functional Theory Investigation. J Phys Chem A 115:7656–7663
Shinohara H, Kotani M (1980) Singlet energy transfer in p-Terphenyl crystal doped with Tetracene. Bull Chem Soc Jpn 53:3171–3175
Mitsui M, Kawano Y (2013) Electronic energy transfer in tetracene-doped p-terphenyl nanoparticles: extraordinarily high fluorescence enhancement and quenching efficiency. Chem Phys 419:30–36
Mane KG, Nagore PB, Pujari SR (2017) Green light emitting tricomponent luminophors of 2-naphthol for construction of organic light emitting. JournalINX 3:38
Pujari SR, Bhosale PN, Rao PMR, Patil SR (2002) Structural and optical studies of perylene-doped polymer thin films. Indian J Pure Appl Phys 40:896
Pujari SR, Jadhav SA, Bhosale PN, Rao PMR, Patil SR (2002) Fluorescence studies of biphenyl doped by pyrene and perylene. Indian J Pure Appl Phys 40:115
Gharge MN, Bhattar SL, Kolekar GB, Patil SR (2008) Structural and photophysical aspects of perylene doped anthracene crystalline powders prepared by microwave heating. Indian J Chem A 47:1642
Cullity BD (1978) Elelments of X-ray Diffraction, 2nd Edn. Addision-Weslye Publshing Company, Inc., USA, p 102
Mane KG, Nagore PB, Pujari SR (2019) Synthesis, Photophysical, electrochemical and thermal study of biphenyl Luminophors: green light emitting materials. J fluor 29:177–183
Shaikh AM, Sharma BK, Chacko S, Kamble RM (2015) Synthesis, Photophysical, Electrochemical and Thermal Studies of Triarylamines based on benzo[g]quinoxalines. J Chem 127:1571
Mane KG, Nagore PB, Pujari SR (2018) Synthesis, Photophysical, electrochemical and thermal investigation of anthracene doped 2-Naphthol Luminophors and their thin films for optoelectronic devices. J Fluoresc 28:1023–1028
Acknowledgements
The authors are thankful to Sophisticated Analytical Instumentation Facility Centre, Indian Institute of Technology, Madras and to the Instrumentation Centre, Solapur University, Solapur, Maharashtra-India.
The authors also express special thanks to Dr. Sutrave and Mr. Sangam Gaikwad of Electronic Dept., D.B.F. Dayanand College of Science, Solapur, Maharashtra – India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mane, K.G., Nagore, P.B. & Pujari, S.R. Synthesis of Highly Fluorescent D-A Based p-Terphenyl Luminophors and their Thin Films for Optoelectronic Applications. J Fluoresc 29, 1001–1006 (2019). https://doi.org/10.1007/s10895-019-02413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02413-0