Abstract
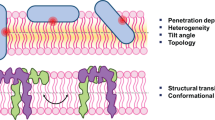
WALPs are prototypical, α-helical transmembrane peptides that represent a consensus sequence for transmembrane segments of integral membrane proteins and serve as excellent models for exploring peptide-lipid interactions and hydrophobic mismatch in membranes. Importantly, the WALP peptides are in direct contact with the lipids. They consist of a central stretch of alternating hydrophobic alanine and leucine residues capped at both ends by tryptophans. In this work, we employ wavelength-selective fluorescence approaches to explore the intrinsic fluorescence of tryptophan residues in WALP23 in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membranes. Our results show that the four tryptophan residues in WALP23 exhibit an average red edge excitation shift (REES) of 6 nm, implying their localization at the membrane interface, characterized by a restricted microenvironment. This result is supported by fluorescence anisotropy and lifetime measurements as a function of wavelength displayed by WALP23 tryptophans in POPC membranes. These results provide a new approach based on intrinsic fluorescence of interfacial tryptophans to address protein-lipid interaction and hydrophobic mismatch.




Similar content being viewed by others
References
Killian JA (2003) Synthetic peptides as models for intrinsic membrane proteins. FEBS Lett 555:134–138
Killian JA, Salemink I, de Planque MRR, Lindblom G, Koeppe II RE, Greathouse DV (1996) Induction of nonbilayer structures in diacylphosphatidylcholine model membranes by transmembrane α-helical peptides: importance of hydrophobic mismatch and proposed role of tryptophans. Biochemistry 35:1037–1045
Vostrikov VV, Koeppe II RE (2011) Response of GWALP transmembrane peptides to changes in the tryptophan anchor positions. Biochemistry 50:7522–7535
Vostrikov VV, Daily AE, Greathouse DV, Koeppe II RE (2010) Charged or aromatic anchor residue dependence of transmembrane peptide tilt. J Biol Chem 285:31723–31730
van’t Hag L, Li X, Meikle TG, Hoffmann SV, Jones NC, Pedersen JS, Hawley AM, Gras SL, Conn CE, Drummond CJ (2016) How peptide molecular structure and charge influence the nanostructure of lipid bicontinuous cubic mesophases: model synthetic WALP peptides provide insights. Langmuir 32:6882–6894
Killian JA (1992) Gramicidin and gramicidin-lipid interactions. Biochim Biophys Acta 1113:391–425
Koeppe II RE, Andersen OS (1996) Engineering the gramicidin channel. Annu Rev Biophys Biomol Struct 25:231–258
Kelkar DA, Chattopadhyay A (2007) The gramicidin ion channel: a model membrane protein. Biochim Biophys Acta 1768:2011–2025
Arkin IT, Brunger AT (1998) Statistical analysis of predicted transmembrane α-helices. Biochim Biophys Acta 1429:113–128
Adamian L, Nanda V, DeGrado WF, Liang J (2005) Empirical lipid propensities of amino acid residues in multispan alpha helical membrane proteins. Proteins 59:496–509
Strandberg E, Ӧzdirekcan S, Rijkers DTS, van der Wel PCA, Koeppe II RE, Liskamp RMJ, Killian JA (2004) Tilt angles of transmembrane model peptides in oriented and non-oriented lipid bilayers as determined by 2H solid-state NMR. Biophys J 86:3709–3721
van der Wel PCA, Strandberg E, Killian JA, Koeppe II RE (2002) Geometry and intrinsic tilt of a tryptophan-anchored transmembrane alpha-helix determined by 2H NMR. Biophys J 83:1479–1488
Schiffer M, Chang C-H, Stevens FJ (1992) The functions of tryptophan residues in membrane proteins. Protein Eng 5:213–214
Kelkar DA, Chattopadhyay A (2006) Membrane interfacial localization of aromatic amino acids and membrane protein function. J Biosci 31:297–302
Mouritsen OG, Bloom M (1984) Mattress model of lipid-protein interactions in membranes. Biophys J 46:141–153
Killian JA (1998) Hydrophobic mismatch between proteins and lipids in membranes. Biochim Biophys Acta 1376:401–416
Dumas F, Lebrun MC, Tocanne J-F (1999) Is the protein/lipid hydrophobic matching principle relevant to membrane organization and functions? FEBS Lett 458:271–277
Andersen OS, Koeppe II RE (2007) Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36:107–130
Rao BD, Shrivastava S, Chattopadhyay A (2017) Hydrophobic mismatch in membranes: when the tail matters. In: Chattopadhyay A (ed) Membrane organization and dynamics. Springer, Heidelberg, pp 375–387
van der Wel PCA, Pott T, Morein S, Greathouse DV, Koeppe II RE, Killian JA (2000) Tryptophan-anchored transmembrane peptides promote formation of nonlamellar phases in phosphatidylethanolamine model membranes in a mismatch-dependent manner. Biochemistry 39:3124–3133
Morein S, Koeppe II RE, Lindblom G, de Kruijff B, Killian JA (2000) The effect of peptide/lipid hydrophobic mismatch on the phase behavior of model membranes mimicking the lipid composition in Escherichia coli membranes. Biophys J 78:2475–2485
de Planque MRR, Bonev BB, Demmers JAA, Greathouse DV, Koeppe II RE, Separovic F, Watts A, Killian JA (2003) Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry 42:5341–5348
de Planque MRR, Kruijtzer JAW, Liskamp RMJ, Marsh D, Greathouse DV, Koeppe II RE, de Kruijff B, Killian JA (1999) Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane α-helical peptides. J Biol Chem 274:20839–20846
de Planque MRR, Boots J-WP, Rijkers DTS, Liskamp RMJ, Greathouse DV, Killian JA (2002) The effects of hydrophobic mismatch between phosphatidylcholine bilayers and transmembrane α-helical peptides depend on the nature of interfacially exposed aromatic and charged residues. Biochemistry 41:8396–8404
Ӧzdirekcan S, Rijkers DTS, Liskamp RMJ, Killian JA (2005) Influence of flanking residues on tilt and rotation angles of transmembrane peptides in lipid bilayers. A solid-state 2H NMR study. Biochemistry 44:1004–1012
Mortazavi A, Rajagopalan V, Sparks KA, Greathouse DV, Koeppe II RE (2016) Juxta-terminal helix unwinding as a stabilizing factor to modulate the dynamics of transmembrane helices. ChemBioChem 17:462–465
Ghosh AK, Rukmini R, Chattopadhyay A (1997) Modulation of tryptophan environment in membrane-bound melittin by negatively charged phospholipids: implications in membrane organization and function. Biochemistry 36:14291–14305
Rawat SS, Kelkar DA, Chattopadhyay A (2004) Monitoring gramicidin conformations in membranes: a fluorescence approach. Biophys J 87:831–843
Chattopadhyay A, Rawat SS, Greathouse DV, Kelkar DA, Koeppe II RE (2008) Role of tryptophan residues in gramicidin channel organization and function. Biophys J 95:166–175
Chaudhuri A, Haldar S, Sun H, Koeppe RE II, Chattopadhyay A (2014) Importance of indole N-H hydrogen bonding in the organization and dynamics of gramicidin channels. Biochim Biophys Acta 1838:419–428
Mukherjee S, Chattopadhyay A (1995) Wavelength-selective fluorescence as a novel tool to study organization and dynamics in complex biological systems. J Fluoresc 5:237–246
Demchenko AP (2008) Site-selective red-edge effects. Methods Enzymol 450:59–78
Haldar S, Chaudhuri A, Chattopadhyay A (2011) Organization and dynamics of membrane probes and proteins utilizing the red edge excitation shift. J Phys Chem B 115:5693–5706
Chattopadhyay A, Haldar S (2014) Dynamic insight into protein structure utilizing red edge excitation shift. Acc Chem Res 47:12–19
Gleason NJ, Vostrikov VV, Greathouse DV, Grant CV, Opella SJ, Koeppe II RE (2012) Tyrosine replacing tryptophan as an anchor in GWALP peptides. Biochemistry 51:2044–2053
Baron CB, Coburn RF (1984) Comparison of two copper reagents for detection of saturated and unsaturated neutral lipids by charring densitometry. J Liq Chromatogr 7:2793–2801
McClare CWF (1971) An accurate and convenient organic phosphorus assay. Anal Biochem 39:527–530
Chen GC, Yang JT (1977) Two-point calibration of circular dichrometer with d-10-camphorsulfonic acid. Anal Lett 10:1195–1207
Chakraborty H, Chattopadhyay A (2017) Sensing tryptophan microenvironment of amyloid protein utilizing walvelength-selective fluorescence approach. J Fluoresc 27:1995–2000
Rao BD, Chakraborty H, Keller S, Chattopadhyay A (2018) Aggregation behavior of pHLIP in aqueous solution at low concentrations: a fluorescence study. J Fluoresc 28:967–973
Lakowicz JR (2006) Principles of fluorescence spectroscopy. 3rd edn. Springer, New York
Kučerka N, Nieh M-P, Katsaras J (2011) Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim Biophys Acta 1808:2761–2771
de Planque MRR, Goormaghtigh E, Greathouse DV, Koeppe II RE, Kruijtzer JAW, Liskamp RMJ, de Kruijff B, Killian JA (2001) Sensitivity of single membrane-spanning α-helical peptides to hydrophobic mismatch with a lipid bilayer: effects on backbone structure, orientation, and extent of membrane incorporation. Biochemistry 40:5000–5010
Mukherjee S, Chattopadhyay A (1994) Motionally restricted tryptophan environments at the peptide-lipid interface of gramicidin channels. Biochemistry 33:5089–5097
Prendergast FG (1991) Time-resolved fluorescence techniques: methods and applications in biology. Curr Opin Struct Biol 1:1054–1059
Pal S, Aute R, Sarkar P, Bose S, Deshmukh MV, Chattopadhyay A (2018) Constrained dynamics of the sole tryptophan in the third intracellular loop of the serotonin1A receptor. Biophys Chem 240:34–41
Acknowledgments
This work was supported by intramural research grants from the Council of Scientific and Industrial Research, Govt. of India. A.C. gratefully acknowledges support from SERB Distinguished Fellowship (Department of Science and Technology, Govt. of India). R.E.K. acknowledges support from MCB grant 1713242 from the United States National Science Foundation. S.P. thanks the University Grants Commission for the award of a Senior Research Fellowship. We thank members of the Chattopadhyay laboratory for their comments and discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 146 kb)
Rights and permissions
About this article
Cite this article
Pal, S., Koeppe, R.E. & Chattopadhyay, A. Wavelength-Selective Fluorescence of a Model Transmembrane Peptide: Constrained Dynamics of Interfacial Tryptophan Anchors. J Fluoresc 28, 1317–1323 (2018). https://doi.org/10.1007/s10895-018-2293-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2293-5