Abstract
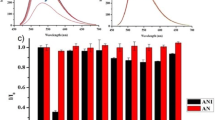
A variety of new coumarin derivatives containing C-4 bridged 2,6-dicyanoanilines (4a-4d) were synthesized via multicomponent one pot approach. These novel sensors were characterized by spectral analysis and a series of pH sensing fluorescence studies were performed, the results indicating that the sensors are highly selective and more effective at various pH. The fluorescence colour changes at different pH could be directly detected by naked eyes.







Similar content being viewed by others
References
Suzuki Y, Yokoyama K (2015) Development of functional fluorescent molecular probes for the detection of biological substances. Biosensors (Basel) 5:337–363
Xiao-Xiang Z, Wang Z, Yue X, Ying M, Dale OK, Chen X (2013) pH-sensitive fluorescent dyes: are they really pH-sensitive in cells? Mol Pharm 10:1910–1917
Desai AS, Chauhan VM, Johnston APR, Esler T, Aylott JW (2013) Fluorescent nanosensors for intracellular measurements: synthesis, characterization, calibration, and measurement. Front Physiol 4:401
Korostynska O, Arshak K, Gill E, Arshak A (2007) Review on state-of-the-art in polymer based pH sensors. Sensors (Basel) 7:3027–3042
Dybko A, Wroblewski W, Rozniecka E, Pozniak K, Maciejewski J, Romaniuk R, Brzozka Z (1998) Assessment of water quality based on multiparameter fiber optic probe. Sens Actuators B Chem 51:208–213
Schirrmann M, Gebbers R, Kramer E, Seidel J (2011) Soil pH mapping with an on-the-go sensor. Sensors 11:573–5985
Bernard V, Berberan-Santos MN. Molecular fluorescence: principles and applications. Wiley doi: 10.1002/9783527650002
Lakowicz JR. Principles of fluorescence spectroscopy. Springer doi: 10.1007/978-0-387-46312-46314
Wu J, Deng A, Jiang W, Tian R, Shen Y (2017) Synthesis and in vitro evaluation of pH-sensitive magnetic nanocomposites as methotrexate delivery system for targeted cancer therapy. Mater Sci Eng C 71:132–140
Borate BH, Kudale SA, Agalave GS (2012) Synthesis of substituted 2,6-Dicyanoanilines and related compounds-a review. Org Prep Proc Int 44:467–521
Cui SL, Lin XF, Wang YG (2005) Parallel synthesis of strongly fluorescent polysubstituted 2,6-dicyanoanilines via microwave-promoted multicomponent reaction. J Org Chem 70:2866–2869
Yalcina E, Kutlua CY, Korkmaza V, Sahinb E, Seferoglua Z (2015) 2,6-Dicyanoaniline based donor-acceptor compounds: the facile synthesis of fluorescent 3,5-diaryl/hetaryl-2,6-dicyanoanilines. ARKIVOC 5:202–218
Albinsson B, Mattias PE, Pettersson K, Winters MU (2007) Electron and energy transfer in donor–acceptor systems with conjugated molecular bridges. Phys Chem Chem Phys 9:5847–5864
Bando P, Martin N, Segura JL, Seoane C (1994) Single-component donor-acceptor organic semiconductors derived from TCNQ. J Org Chem 59:4618–4629
Chu CW, Ouyang J, Tseng JH, Yang Y (2005) Organic Donor–Acceptor System Exhibiting Electrical Bistability for Use in Memory Devices. Adv Mate 17:1440–1443
Lakowicz JR (1999) Principles of fluorescence spectroscopy. Kluwer Academic/Plenum Publishers, New York, p 280
Valeur B (2001) Molecular fluorescence: Principles and Aplications. Wiley-Verlag Chemie GmbH, Weinheim, p 79
de Prasanna SA, Gunaratante HQN, Gunnlaugsson TH, Huxley AJM, Mc Coy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
de Prasanna SA, Vance TP, West MES, Wright GD (2008) Bright molecules with sense, logic, numeracy and utility. Org Biomol Chem 6:2468–2481
Soutar I (1991) The application of luminescence technique in polymer science. Polym Int 26:35–49
Morawetz H (1999) On the versatility of fluorescence technique in polymer research. J Polym Sci A Polym Chem 37:1725–1735
Sarker AM, Kaneko Y, Neckers DC (1998) Photochemistry and photophysics of novel photoinitiators: N,N,N-Tributyl-N-(4-methylene-7-methoxycoumarin) ammonium borates. J Photochem Photobiol 117:67–74
Acceta R, Corradini R, Marcelli R (2011) Enantioselective sensing by luminescence. Top Curr Chem 300:175–216
Mariusz T, Kim D, Singha S, Krzeszewski M, Daniel KHA, Gryko T (2015) p-Expanded coumarins: synthesis, optical properties and applications. J Mater Chem 3:1421–1446
Takuya S, Toshiki S, Hiroyuki K, Tomoya H (2014) Development of a novel fluorescent sensor to detect a specific range of pH. Tett Lett 55:6784–6786
Na’il S, Yaseen AA, Werner MN (2008) Novel fluorescent pH sensor based on coumarin with piperazine and imidazole substituents. Spectrochim Acta A Mol Biomol Spectrosc 71:818–822
Rashmi CK, Samundeeswari S, Bahubali MC, Megharaja H, Manohar VK, Lokesh AS (2016) A one pot green synthetic route for construction of coumarin C-4 bridged 2, 6-dicyanoanilines and their photophysical study. Synth Comm 46:2063–2072
Acknowledgements
We acknowledge the University Grant Commission (UGC) and also UGC-UPE New Delhi, for the financial support. Authors also thank NMR Research Center, Indian Institute of Science (I.I.Sc.), Bangalore and University Sophisticated Instrumentation Center, Dharwad (USIC) for spectral analysis. One of the authors Rashmi C. Kulkarni expresses her gratitude towards Lohit Naik for his advice and insights.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 675 kb)
Rights and permissions
About this article
Cite this article
Kulkarni, R.C., Samundeeswari, S., Shaikh, F. et al. Synthesis of Naked-eye Detectable Fluorescent 2H-chromen-2-One 2, 6-Dicyanoanilines: Effect of Substituents and pH on Its Luminous Behavior. J Fluoresc 27, 1613–1619 (2017). https://doi.org/10.1007/s10895-017-2098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2098-y