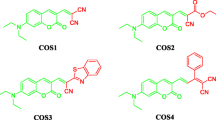
Abstract
The coumarin molecules with 7-(N,N-diethylamino) substitution and aryl azo (Ar–N=N-) at 3-position were synthesized, by reacting diazonium salt of substituted amines and 7-(N, N-diethylamino)-4-hydroxy coumarin under basic conditions. They were found to be fluorescent despite the presence of azo group. The azo group rotation was blocked by complexing with -BF2, so as to get a red shift in absorption. The azo molecules show charge transfer, whereas BF2-complexes do not. The dipole moment ratios between the ground and excited states calculated suggest highly polar excited state and an intra-molecular charge transfer at the excited state in the case of azo dyes. The NLO properties were calculated by solvatochromic method and computationally. Second order hyperpolarizability was found to be 46 to 1083 times more than urea. DFT and TDTDF calculations were performed to understand the electronic properties of the molecules at the ground as well as excited states.








Similar content being viewed by others
References
Cigáň M, Donovalová J, Szöcs V et al (2013) 7-(Dimethylamino)coumarin-3-carbaldehyde and its phenylsemicarbazone: TICT excited state modulation, fluorescent H-aggregates, and preferential solvation. J Phys Chem A 117:4870–4883. doi:10.1021/jp402627a
Krzeszewski M, Vakuliuk O, Gryko DT (2013) Color-tunable fluorescent dyes based on benzo[c]coumarin. Eur J Org Chem 5631–5644. doi:10.1002/ejoc.201300374
Azzouz IM, Salah A (2012) Nonlinear optical absorption and NIR to blue conversion in highly stable polymeric dye rod. Appl Phys B 108:469–474. doi:10.1007/s00340-012-4915-y
Chen J, Liu W, Zhou B et al (2013) Coumarin- and rhodamine-fused deep red fluorescent dyes: synthesis, photophysical properties, and bioimaging in vitro. J Org Chem 78:6121–6130. doi:10.1021/jo400783x
Nedumpara RJ, Thomas KJ, Jayasree VK et al (2007) Study of solvent effect in laser emission from Coumarin 540 dye solution. Appl Opt 46:4786. doi:10.1364/AO.46.004786
Christie RM, Morgan KM, Islam MS (2008) Molecular design and synthesis of N-arylsulfonated coumarin fluorescent dyes and their application to textiles. Dye Pigment 76:741–747. doi:10.1016/j.dyepig.2007.01.018
Kim T-K, Lee D-N, Kim H-J (2008) Highly selective fluorescent sensor for homocysteine and cysteine. Tetrahedron Lett 49:4879–4881
Keskin SS, Aslan N, Bayrakçeken F (2009) Optical properties and chemical behavior of Laser-dye Coumarin-500 and the influence of atmospheric corona discharges. Spectrochim Acta A Mol Biomol Spectrosc 72:254–259. doi:10.1016/j.saa.2008.09.024
Painelli A, Terenziani F (2001) Linear and non-linear optical properties of push–pull chromophores: vibronic and solvation effects beyond perturbation theory. Synth Met 124:171–173. doi:10.1016/S0379-6779(01)00431-3
Jung HS, Kwon PS, Lee JWJHJY et al (2009) Coumarin-derived Cu(2+)-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012. doi:10.1021/ja808611d
Sheng R, Wang P, Gao Y et al (2008) Colorimetric test kit for Cu2+ detection. Org Lett 10:5015–5018. doi:10.1021/ol802117p
Yazdanbakhsh MR, Ghanadzadeh A, Moradi E (2007) Synthesis of some new azo dyes derived from 4-hydroxy coumarin and spectrometric determination of their acidic dissociation constants. J Mol Liq 136:165–168. doi:10.1016/j.molliq.2007.03.005
Shahinian EGH, Haiduc I, Sebe I (2011) Synthesis and characterization of new azo coumarin dyes. UPB Sci Bull Ser B Chem Mater Sci 73:153–160
Morlet-Savary F, Ley C, Jacques P, Fouassier JP (2001) Photophysics of a bridged 7-diethylamino-4-methyl-coumarin C102: studying the hydrogen bonding effect by time resolved stimulated emission. J Phys Chem A 105:11026–11033
Griffiths J, Millar V, Bahra G (1995) The influence of chain length and electron acceptor residues in 3-substituted 7- N, N-diethylaminocoumarin dyes. Dye Pigment 28:327–339
Yang Y, Hughes RP, Aprahamian I (2012) Visible light switching of a BF2-coordinated azo compound. J Am Chem Soc 134:15221–15224. doi:10.1021/ja306030d
Li Y, Patrick BO, Dolphin D (2009) Near-Infrared absorbing azo dyes: synthesis and x-ray crystallographic and spectral characterization of monoazopyrroles, bisazopyrroles, and a boron-azopyrrole complex. J Org Chem 74:5237–5243. doi:10.1021/jo9003019
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. doi:10.1103/PhysRev.140.A1133
Menzel R, Ogermann D, Kupfer S et al (2012) 4-Methoxy-1,3-thiazole based donor-acceptor dyes: characterization, X-ray structure, DFT calculations and test as sensitizers for DSSC. Dye Pigment 94:512–524. doi:10.1016/j.dyepig.2012.02.014
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Tablet C, Minea L, Dumitrache L, Hillebrand M (2012) Experimental and theoretical study of the inclusion complexes of 3-carboxycoumarin acid with β- and 2-hydroxypropyl-β-cyclodextrins. Spectrochim Acta A Mol Biomol Spectrosc 92:56–63. doi:10.1016/j.saa.2012.02.027
Rajeev S, Husain MM (2011) Solvent effect on coumarin dye: calculation of ground and excited state dipole moments. J Indian Chem Soc 88:1541–1546
Deshmukh MS, Sekar N (2014) A combined experimental and TD-DFT investigation of three disperse azo dyes having the nitroterephthalate skeleton. Dye Pigment 103:25–33. doi:10.1016/j.dyepig.2013.10.035
Wong MW, Frisch MJ, Wiberg KB (1991) Solvent effects. 1. The mediation of electrostatic effects by solvents. J Am Chem Soc 113:4776–4782. doi:10.1021/ja00013a010
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, revision C.01. Gaussian 09, revis. B.01. Gaussian, Inc, Wallingford
Dennington R, Keith TMJ (2009) Gaussview
Knierzinger A, Wolfbeis OS (1980) Syntheses of fluorescent dyes. IX. New 4-hydroxycoumarins, 4-hydroxy-2-quinolones, 2H,5H-Pyrano[3,2-c]benzopyran-2,5-diones and 2H,5H-Pyrano[3,2-c]quinoline-2,5-diones. J Heterocycl Chem 17:225–229. doi:10.1002/jhet.5570170204
Ozen AS, Doruker P, Aviyente V (2007) Effect of cooperative hydrogen bonding in azo-hydrazone tautomerism of azo dyes. J Phys Chem A 111:13506–13514. doi:10.1021/jp0755645
Qu J, Liu B, He J (2014) Photofading mechanisms of azo dye in its azo and hydrazone forms under UV irradiation. J Chem Pharm Res 6:1149–1154
Umape PG, Patil VS, Padalkar VS et al (2013) Synthesis and characterization of novel yellow azo dyes from 2-morpholin-4-yl-1,3-thiazol-4(5H)-one and study of their azo–hydrazone tautomerism. Dye Pigment 99:291–298. doi:10.1016/j.dyepig.2013.05.002
Lippert E (1957) Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Z Elektrochem Ber Bunsenges Phys Chem 61:962–975. doi:10.1002/bbpc.19570610819
Valeur B (2001) Molecular fluorescence. doi:10.1002/3527600248
Bilot L, Kawski A (1962) Zur Theorie des Einflusses von Lösungsmitteln auf die Elektronenspektren der Moleküle. Zeitschrift Naturforsch. Tl. A 17
Bakhshiev NG (1972) Spectroscopy of intermolecular interactions (in Russian). Isd. Nauka, Leningrad
Liptay W (1965) Die Lösungsmittelabhängigkeit der Wellenzahl von Elektronenbanden und die chemisch-physikalischen Grundlagen. Z Naturforsch A 20a:1441–1447
Turro NJ (1978) Modern molecular photochemistry. Benjamin-Cummings, New York
Coe BJ, Harris JA, Asselberghs I et al (2002) Quadratic nonlinear optical properties ofN-Aryl stilbazolium dyes. Adv Funct Mater 12:110–116. doi:10.1002/1616-3028(20020201)12:2<110::AID-ADFM110>3.0.CO;2-Y
Sharafudeen KN, Adithya A, Vijayakumar S et al (2011) Multiphoton absorption process and self-focusing effect in coumarin derivative doped PMMA films by Z-scan and optical limiting studies. Curr Appl Phys 11:1089–1093. doi:10.1016/j.cap.2011.02.001
Sun YF, Wang HP, Chen ZY, Duan WZ (2013) Solid-state fluorescence emission and second-order nonlinear optical properties of coumarin-based fluorophores. J Fluoresc 23:123–130. doi:10.1007/s10895-012-1125-2
Raj RK, Gunasekaran S, Gnanasambandan T, Seshadri S (2015) Combined spectroscopic and DFT studies on 6-bromo-4-chloro-3-formyl coumarin. Spectrochim Acta A Mol Biomol Spectrosc 139:505–514. doi:10.1016/j.saa.2014.12.024
Abbotto A, Beverina L, Bradamante S et al (2003) A distinctive example of the cooperative interplay of structure and environment in tuning of intramolecular charge transfer in second-order nonlinear optical chromophores. Chem - A Eur J 9:1991–2007. doi:10.1002/chem.200204356
Oudar JL, Zyss J (1982) Structural dependence of nonlinear-optical properties of methyl-(2,4-dinitrophenyl)-aminopropanoate crystals. Phys Rev A 26:2016–2027. doi:10.1103/PhysRevA.26.2016
Morley JO, Docherty VJ, Pugh D (1987) Non-linear optical properties of organic molecules. Part 2. Effect of conjugation length and molecular volume on the calculated hyperpolarisabilities of polyphenyls and polyenes. J Chem Soc Perkin Trans 2:1351. doi:10.1039/p29870001351
Meshulam G, Kotler Z, Berkovic G (2002) Time-resolved electric-field-induced second harmonic: simultaneous measurement of first and second molecular hyperpolarizabilities. Opt Lett 27:1132–1134. doi:10.1364/OL.27.001132
Heesink GJT, Ruiter AGT, Van Hulst NF, Bölger B (1993) Determination of hyperpolarizability tensor components by depolarized hyper Rayleigh scattering. Phys Rev Lett 71:999–1002. doi:10.1103/PhysRevLett.71.999
Oudar JL, Chemla DS (1977) Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J Chem Phys 66:2664. doi:10.1063/1.434213
Carlotti B, Flamini R, Kikaš I et al (2012) Intramolecular charge transfer, solvatochromism and hyperpolarizability of compounds bearing ethenylene or ethynylene bridges. Chem Phys 407:9–19. doi:10.1016/j.chemphys.2012.08.006
Paley MS, Harris JM, Looser H et al (1989) A solvatochromic method for determining second-order polarizabilities of organic molecules. J Org Chem 54:3774–3778. doi:10.1021/jo00277a007
McRae EG (1957) Theory of solvent effects on molecular electronic spectra. Frequency shifts. J Phys Chem 61:562–572. doi:10.1021/j150551a012
Bruni S, Cariati E, Cariati F et al (2001) Determination of the quadratic hyperpolarizability of trans-4-[4-(dimethylamino)styryl]pyridine and 5-dimethylamino-1,10-phenanthroline from solvatochromism of absorption and fluorescence spectra: a comparison with the electric-field-induced second-harmon. Spectrochim Acta A Mol Biomol Spectrosc 57:1417–1426. doi:10.1016/S1386-1425(00)00483-2
Oudar JL (1977) Optical nonlinearities of conjugated molecules. Stilbene derivatives and highly polar aromatic compounds. J Chem Phys 67:446–457. doi:10.1063/1.434888
De Paris R (1982) Nonlinear-optical properties. Phys Rev A 26:2016–2027
Kwon OP, Jazbinsek M, Seo JI et al (2010) First hyperpolarizability orientation in asymmetric pyrrole-based polyene chromophores. Dye Pigment 85:162–170. doi:10.1016/j.dyepig.2009.10.019
Weaver CS, Smith SW, Hyndman RD et al (1991) + 0.028. Science 252:103–106
Cheng L-T, Tam W, Stevenson SH et al (1991) Experimental investigations of organic molecular nonlinear optical polarizabilities. 1. Methods and results on benzene and stilbene derivatives. J Phys Chem 95:10631–10643. doi:10.1021/j100179a026
Dirk CW, Cheng L-T, Kuzyk MG (1992) A simplified three-level model describing the molecular third-order nonlinear optical susceptibility. Int J Quantum Chem 43:27–36. doi:10.1002/qua.560430106
Kuzyk MG, Dirk CW (1990) Effects of centrosymmetry on the nonresonant electronic third-order nonlinear optical susceptibility. Phys Rev A 41:5098–5109. doi:10.1103/PhysRevA.41.5098
Acknowledgments
Abhinav Tathe is thankful to University Grants Commission, New Delhi (India) for award of Junior and Senior Research fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1829 kb)
Rights and permissions
About this article
Cite this article
Tathe, A.B., Sekar, N. Red Emitting Coumarin—Azo Dyes : Synthesis, Characterization, Linear and Non-linear Optical Properties-Experimental and Computational Approach. J Fluoresc 26, 1279–1293 (2016). https://doi.org/10.1007/s10895-016-1815-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1815-2