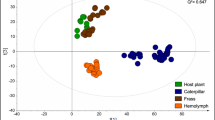
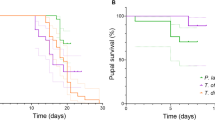
Abstract
Microplitis croceipes is a solitary parasitoid that specializes on noctuid larvae of Helicoverpa zea and Heliothis virescens. Both the parasitoid and its hosts are naturally distributed across a large part of North America. When parasitoids deposit their eggs into hosts, venom and polydnaviruses (PDVs) are also injected into the caterpillars, which can suppress host immune responses, thus allowing parasitoid larvae to develop. In addition, PDVs can regulate host oral cues, such as glucose oxidase (GOX). The purpose of this study was to determine if parasitized caterpillars differentially induce plant defenses compared to non-parasitized caterpillars using two different caterpillar host/plant systems. Heliothis virescens caterpillars parasitized by M. croceipes had significantly lower salivary GOX activity than non-parasitized caterpillars, resulting in lower levels of tomato defense responses, which benefited parasitoid performance by increasing the growth rate of parasitized caterpillars. In tobacco plants, parasitized Helicoverpa zea caterpillars had lower GOX activity but induced higher plant defense responses. The higher tobacco defense responses negatively affected parasitoid performance by reducing the growth rate of parasitized caterpillars, causing longer developmental periods, and reduced cocoon mass and survival of parasitoids. These studies demonstrate a species-specific effect in different plant-insect systems. Based on these results, plant perception of insect herbivores can be affected by parasitoids and lead to positive or negative consequences to higher trophic levels depending upon the particular host-plant system.








Similar content being viewed by others
References
Acevedo FE, Rivera-Vega LJ, Chung SH, Ray S, Felton GW (2015) Cues from chewing insects-the interaction of DAMPs, HAMPs, MAMPs and effectors. Curr Opin Plant Biol 26:80–86
Asgari S (2006) Venom protein from polydnavirus-producing endoparasitoids: their role in host-parasite interaction. Arch Insect Biochem Physiol 61:146–156
Asgari S, Rivers DB (2011) Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annu Rev Entomol 56:313–335
Ashley TR (1986) Geographical distribution and parasitization levels for parasitoids of the fall armyworm, Spodoptera frugiperda. Fla Entomol 69:516–524
Ashley TR, Barfield CS, Waddill VH, Mitchell ER (1983) Parasitization of fall armyworm larvae on volunteer corn, bermudagrass, and paragrass. Fla Entomol 66:267–271
Bede JC, Musser RO, Felton GW, Korth KL (2006) Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid bionsynthesis. Plant Mol Biol 60:519–531
Bitra K, Zhang S, Strand MR (2011) Transcriptomic profiling of Microplitis demolitor bracovirus reveals host, tissue and stage-specific patterns of activity. J Gen Virol 92:2060–2071
Bosak EJ (2011) Using a developmental comparison to decipher priming of induced defenses in maize and its effects on a generalist herbivore. PhD thesis, The Pennsylvania State University, University Park, USA
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burke GR, Strand MR (2012) Polydnaviruses of parasite wasps: domestication of viruses to act as gene delivery vectors. Insects 3:91–119
Chung SH, Rosa C, Scully ED, Peiffer M, Tooker JF, Hoover K, Luthe DS, Felton GW (2013) Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci U S A 13:15728–15733
Cusumano A, Zhu F, Volkoff A-N, Verbaarschot P, Bloen J, Vogel H, Dicke M, Poelman EH (2018) Parasitic wasp-associated symbiont affects plant-mediated species interactions between herbivores. Ecol Lett 21:957–968
Diezel C, von Dahl CC, Gaquerel E, Baldwin IT (2009) Different Lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol 150:1576–1586
Eichenseer H, Mathews MC, Bi JL, Murphy JB, Felton GW (1999) Salivary glucose oxidase: multifunctional roles for Helicoverpa zea? Arch Insect Biochem Physiol 42:99–109
Eichenseer H, Mathews MC, Powell JS, Felton GW (2010) Survey of a salivary effector in caterpillars: glucose oxidase variation and correlation with host range. J Chem Ecol 36:885–897
Fathpour H, Dahlman DL (1995) Polydnavirus of Microplitis croceipes prolongs the larval period and changes hemolymph protein content of the host, Heliothis virescens. Arch Insect Biochem 28:33–48
Felton GW, Donato K, Del Vecchio RJ, Duffey SS (1989) Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J Chem Ecol 15:2667–2694
Fitt GP (1989) The ecology of Helopthis species in relation to agroecosystems. Annu Rev Entomol 34:17–52
Hegazi EM, Abol Ella SM, Bazzaz A, Khamis O, Abo Abd-Allah LMZ (2005) The calyx fluid of Microplitis rufiventris parasitoid and growth of its host Spodoptera littoralis larvae. J Insect Physiol 51:777–787
Hopper KR, King EG (1984) Preference of Microplitis croceipes (Hymenoptera: Braconidae) for instars and species of Heliothis (Lepidoptera: Noctuidae). Environ Entomol 13:1145–1150
King E, Coleman R (1989) Potential for biological control of Heliothis species. Annu Rev Entomol 34:53–75
Lewis WJ, Snow JW (1971) Fecundity, sex rations, and egg distribution by Microplitis croceipes, a parasite of Heliothis. J Econ Entomol 64:6–8
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
López-Uribe MM, Fitzgerald A, Simone-Finstrom M (2017) Inducible versus constitutive social immunity: examining effects of colony infection on glucose oxidase and defensin-1 production in honeybees. R Soc Open Sci 4:170224
Louis J, Peiffer M, Ray S, Luthe DS, Felton GW (2013) Host-specific salivary elicitor(s) of European corn borer induce defenses in tomato and maize. New Phytol 199:66–73
Mohan S, Ma PWK, Williams WP, Luthe DS (2008) A naturally occurring plant cysteine protease possesses remarkable toxicity against insect pests and synergizes Bacillus thuringiensis toxin. PLosOne 3:e1786
Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Caterpillar saliva beats plant defenses. Nature 416:599–560
Musser RO, Cipollini DF, Hum-Musser SM, Williams SA, Brown JK, Felton GW (2005) Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in solanaceous plants. Arch Insect Biochem Physiol 58:128–137
Neunzig HH (1963) Wild host plants of the corn earworm and the tobacco budworm in eastern North Carolina. J Econ Entomol 56:135–139
Ode PJ, Harvey JA, Reichelt M, Gershenzon J, Gols R (2015) Differential induction of plant chemical defense by parasitized and unparasitized herbivores: consequences for reciprocal multitrophic interactions. Oikos 125:1398–1407
Peiffer M, Felton GW (2005) The host plant as a factor in the synthesis and secretion of salivary glucose oxidase in larval Helicoverpa zea. Arch Insect Biochem Physiol 58:106–113
Peterson JA, Ode PJ, Oliveira-Hofman C, Harwood JD (2016) Integration of plant defense traits with biological control of arthropod pests: challenges and opportunities. Front Plant Sci 7:1794. https://doi.org/10.3389/fpls.2016.01794
Poleman EH, Zheng SJ, Zhang Z, Heenskerk NM, Cortesero AM, Dicke M (2011) Parasitoid-specific induction of plant responses to parasitized herbivores affects colonization by subsequent herbivores. Proc Natl Acad Sci U S A 108:19647–19652
Razmi M, Karimpour Y, Safaralizadeh M, Safavi S (2011) Parasitoid complex of cabbage large white butterfly Pieris brassicae (L.) (Lepidoptera, Pieridae) in Urmia with new records from Iran. J Plant Prot Res 51:248–251
Rivera-Vega LJ, Acevedo F, Felton GW (2017) Genomics of Lepidoptera saliva reveals function in herbivory. Curr Opin Insect Sci 19:61–69
Rivera-Vega LJ, Stanley BA, Stanley A, Felton GW (2018) Proteomic analysis of labial saliva of the generalist cabbage looper (Trichoplusia ni) and its role in interactions with host plants. J Insect Physiol 107:97–103
Shikano I, Rosa C, Tan C-W, Felton GW (2017) Tritrophic interactions: microbe-mediated plant effects on insect herbivores. Annu Rev Phytopathol 55:313–331
Smith JW, King EG, Bell JV (1976) Parasites and pathogens among Heliothis species in the Central Mississippi Delta. Environ Entomol 5:224–226
Stadelbacher EA, Powell JE, King EG (1984) Parasitism of Heliothis zea and H. virescens (Lepidoptera: Noctuidae) larvae in wild and cultivated host plants in the Delta of Mississippi. Environ Entomol 13:1167–1172
Tan C-W, Peiffer M, Hoover K, Rosa C, Acevedo FE, Felton GW (2018) Symbiotic polydnavirus of a parasite manipulates caterpillar and plant immunity. Proc Natl Acad Sci U S A 115:5199–5204
Tanaka T, Vinson SB (1991) Interaction of venoms with the calyx fluids of three parasitoids, Cardiochiles nigriceps, Microplitis croceipes (Hymenoptera: Braconidae), and Campoletis sonorensis (Hymenoptera: Ichneumonidae) in effecting a delay in the pupation of Heliothis virescens (Lepidoptera: Noctuidae). Ann Entomol Soc Am 84:87–92
Tian D, Peiffer M, Shoemark E, Tooker J, Haubrug E, Francis F, Luthe DS, Felton GW (2012) Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS One 7:e36168
Tipping PW, Holko CA, Bean RA (2005) Helicoverpa zea (Lepidoptera: Noctuidae) dynamics and parasitism in Maryland soybeans. Fla Entomol 88:55–60
Wang J, Peiffer M, Hoover K, Rosa C, Zeng R, Felton GW (2017) Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New Phytol 214:1294–3106
Wang J, Yang M, SongY AFE, Hoover K, Zeng R, Felton GW (2018) Gut-associated bacteria of Helicoverpa zea indirectly trigger plant defense in maize. J Chem Ecol 44:690–699
Young J, Price R (1975) Incidence, parasitism, and distribution patterns of Heliothis zea on sorghum, cotton, and alfalfa for southwestern Oklahoma. Environ Entomol 4:777–779
Zhu F, Broekgaarden C, Weldegergis BT, Harvey JA, Vosman B, Dicke M, Poelman EH (2015) Parasitism overrides herbivore identity allowing hyperparasitoids to locate their parasitoid host using herbivore-induced plant volatiles. Mol Ecol 24:2886–2899
Zhu F, Cusumano A, Bloem J, Weldegregris BT, Villela A, Fatouros NE, van Loon JJA, Dick M, Harvey JA, Vogel H, Poelman EH (2018) Symbiotic polydnavirus and venom reveal parasitoid to its hyperparasitoids. Proc Natl Acad Sci U S A 115:5205–5210
Zong N, Wang CZ (2004) Induction of nicotine in tobacco by herbivory and its relation to glucose oxidase activity in the labial gland of three noctuid caterpillars. Chin Sci Bull 49:1596–1601
Acknowledgements
We thank Dr. Henry Fadamiro (Auburn University) for providing M. croceipes pupae; Ju-Che Lo for assistance with the experiment and maintaining insect colonies. This research was supported by National Science Foundation Grant IOS-1645548.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, CW., Peiffer, M., Hoover, K. et al. Parasitic Wasp Mediates Plant Perception of Insect Herbivores. J Chem Ecol 45, 972–981 (2019). https://doi.org/10.1007/s10886-019-01120-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-019-01120-1