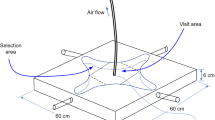
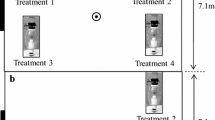
Abstract
The deployment of odor-baited tools for sampling and controlling malaria vectors is limited by a lack of potent synthetic mosquito attractants. A synthetic mixture of chemical compounds referred to as “the Mbita blend” (MB) was shown to attract as many host-seeking malaria mosquitoes as attracted to human subjects. We hypothesized that this effect could be enhanced by adding one or more attractive compounds to the blend. We tested changes in the capability of MB (ammonia + L-lactic acid + tetradecanoic acid +3-methyl-1-butanol + carbon dioxide) to attract host-seeking malaria mosquitoes by addition of selected dilutions of butyl-2-methylbutanoate (1:10,000), 2-pentadecanone (1:100), 1-dodecanol (1:10,000), and butan-1-amine (1:10,000,000). The experiments were conducted in semi-field enclosures and in a village in western Kenya. In semi-field enclosures, the attraction of Anopheles gambiae sensu stricto females to MB-baited traps was not enhanced by adding butyl-2-methylbutanoate. There was, however, an increase in the proportion of An. gambiae caught in traps containing MB augmented with the selected dilutions of butan-1-amine, 2-pentadecanone, and 1-dodecanol. When tested in the village, addition of butan-1-amine to MB enhanced catches of female An. gambiae sensu lato, An. funestus, and Culex mosquitoes. 1-Dodecanol increased attraction of An. gambiae s.l. to the MB, while addition of 2-pentadecanone improved trap catches of An. funestus and Culex mosquitoes. This study demonstrates the possibility of enhancing synthetic odor blends for trapping the malarial mosquitoes An. gambiae s.l. and An. funestus, as well as some culicine species. The findings provide promising results for the optimization and utilization of synthetic attractants for sampling and controlling major disease vectors.


Similar content being viewed by others
References
Bayoh MN, Mathias D, Odiere M, Mutuku F, Kamau L, Gimnig J, Vulule J, Hawley W, Hamel M, Walker E (2010) Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J 9:62
Bukhari T, Takken W, Andrew KG, Koenraadt JMC (2011) Efficacy of aquatain, a monomolecular film for the control of malaria vectors in rice paddies. PLoS One 6:e21713
Charlwood JD, Tomás EV, Egyir-Yawson A, Kampango AA, Pitts RJ (2012) Feeding frequency and survival of Anopheles gambiae in a rice-growing area in Ghana. Med Vet Entomol 26:263–270
Costantini C, Gibson G, Sagnon N, Della Torre A, Brady J, Coluzzi M (1996) Mosquito responses to carbon dioxide in a west African Sudan savanna village. Med Vet Entomol 10:220–227
Dekker T, Steib B, Cardé RT, Geier M (2002) L-lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol 16:91–98
Dia I, Diallo D, Duchemin J, Konate YB, Costantini C, Diallo M (2005) Comparisons of human-landing catches and odor-baited entry traps for sampling malaria vectors in Senegal. J Med Entomol 42:104–109
Durnez L, Coosemans M (2013) Residual transmission of malaria: An old issue for new approaches, pp. 671–704, Anopheles mosquitoes-New insights into malaria vectors. Intech
Gillies MT, Coetzee M (1987) A supplement to the anophelinae of Africa south of the Sahara (Afrotropical region), Johannesburg. S Afr Inst Med Res 55:1–143
Hiscox A, Maire N, Kiche I, Silkey M, Homan T, Oria P, Mweresa CK, Otieno B, Ayugi M, Bousema T, Sawa P, Alaii J, Smith T, Leeuwis C, Mukabana WR, Takken W (2012) The SolarMal project: innovative mosquito trapping technology for malaria control. Malar J 11:45
Jawara M, Smallegange RC, Jeffries D, Nwakanma DC, Awolola TS, Knols BGJ, Takken W, Conway DJ (2009) Optimizing odor-baited trap methods for collecting mosquitoes during the malaria season in the Gambia. PLoS One 4:e8167
Kline DL (2006) Traps and trapping techniques for adult mosquito control. Am Mosq Control Assoc 22:490–496
Kline D (2007) Semiochemicals, traps/targets and mass trapping technology for mosquito management. Am Mosq Control Assoc 23:241–251
Kweka JE, Mahande AN (2009) Comparative evaluation of four mosquitoes sampling methods in rice irrigation schemes of lower Moshi, northern Tanzania. Malar J 8:149
Kweka EJ, Eunice AO, Beda JM, Aneth MM, Mramba N, Franklin M (2011) The role of cow urine in the oviposition site preference of culicine and Anopheles mosquitoes. Parasit Vectors 4:184
Lindh MJ, Okal MN, Herrera-Varela M, Borg-Karlson A, Torto B, Lindsay SW, Fillinger U (2015) Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex. Malar J 14:119
Logan JG (2008) Why do mosquitoes “choose” to bite some people more than others. outlooks on pest man 14:280–283
Logan JG, Birkett MA, Clark SJ, Powers S, Seal NJ, Wadhams LJ, Mordue AJ, Pickett JA (2008) Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol 34:308–322
Mathenge EM, Misiani GO, Irungu LW, Ndegwa PN, Smith LW, Killeen GF, Knols BGJ (2005) Comparative performance of the Mbita trap, CDC light trap and the human landing catch in the sampling of Anopheles arabiensis, An. funestus and culicine species in a rice irrigation in western Kenya. Malar J 4:7
Mboera LEG (2005) Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzan Health Res Bull 7:117–124
Mendis K, Rietvelda A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH (2009) From malaria control to eradication: the WHO perspective. Trop Med & Internl Hlth 14:802–809
Menger DJ, Otieno B, de Rijk M, Mukabana WR, van Loon JJ, Takken W (2014) A push-pull system to reduce house entry of malaria mosquitoes. Malar J 13:119
Menger D, Omusula P, Holdinga M, Homan T, Carreira A, Vandendaele P, Derycke J, Mweresa CK, Mukabana WR, Van Loon JJA, Takken W (2015) Field evaluation of a push-pull system to reduce malaria transmission. PLoS One 10:e0123415
Menger DJ, Omusula P, Wouters K, Oketch C, Carreira AS, Durka M, Derycke JL, Loy DE, Hahn BH, Mukabana WR, Mweresa CK, van Loon JJ, Takken W, Hiscox A (2016) Eave screening and push-pull tactics to reduce house entry by vectors of malaria. AmJTrop Med Hyg 94:868–878
Mukabana WR, Mweresa CK, Otieno B, Omusula P, Smallegange RC, Van Loon JJA, Takken W (2012a) A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J Chem Ecol 38:235–244
Mukabana WR, Mweresa CK, Otieno B, Omusula P, Orindi B, Smallegange RC, Van Loon JJA, Takken W (2012b) Evaluation of low density polyethylene and nylon for delivery of synthetic mosquito attractants. Parasit Vectors 50:123–133
Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C (2013) The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya Parasit Vectors 6
Mweresa CK, Omusula P, Otieno B, Weldegergis BT, Verhulst NO, Dicke M, Van Loon JJA, Takken W, Mukabana RW (2015) Understanding the long-lasting attraction of malaria mosquitoes to odor baits. PLoS One 10:e0121533
Ndiath M, Mazenot C, Gaye A, Konate L, Bouganali C, Faye O, Sokhna C, Trape JF (2011) Methods to collect Anopheles mosquitoes and evaluate malaria transmission: a comparative study in two villages in Senegal. Malar J 10:270
Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, Mbeyela E, Titus E, Munk C, Ngonyani H, Takken W, Mshinda H, Mukabana WR, Moore SJ (2010a) Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS One 5:e8951
Okumu FO, Biswaro L, Mbeleyela E, Killeen GF, Mukabana RW, Moore SJ (2010b) Using nylon strips to dispense mosquito attractants for sampling the malaria vector Anopheles gambiae s.s. J Med Entomol 47:274–282
Okumu FO, Madumla E, John A, Lwetoijera D, Sumaye R (2010c) Attracting, trapping and killing disease-transmitting mosquitoes using odor-baited stations - the Ifakara odor-baited stations. Parasit Vectors 3:12
Pickett JA, Birkett MA, Dewhirst SY, Logan JG, Omolo MO, Torto B, Pelletier J, Syed Z, Leal WS (2010) Chemical ecology of animal and human pathogen vectors in a changing global climate. J Chem Ecol 36:113–121
Qiu YT, Van Loon JJA (2010) Olfactory physiology of blood-feeding vector mosquitoes. Ecol Control Vector-borne Dis 2:39–61
Qiu YT, Smallegange RC, Braak CJF, Spitzen J, Van Loon JJA, Jawara M, Milligan P, Galimard AM, Van Beek TA, Knols BGJ, Takken W (2007) Attractiveness of MM-X traps baited with human or synthetic odor to mosquitoes (Diptera: Culicidae) in the Gambia. J Med Entomol 44:970–983
Qiu YT, Smallegange RC, Van Loon JJA, Takken W (2011) Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Med Vet Entomol 25:247–255
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SF, Killeen GF (2011) Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J 10:80
Schmied W, Takken W, Killeen GF, Knols BGJ, Smallegange RC (2008) Evaluation of two counterflow traps for testing behaviour-mediating compounds for the malaria vector Anopheles gambiae s.s. under semi-field conditions in Tanzania. Malar J 7:230
Scott TW, Takken W (2012) Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol 28:114–121
Smallegange RC, Takken W (2010) Host-seeking behaviour of mosquitoes: responses to olfactory stimuli in the laboratory. Ecol Contl Vector-borne Dis 2:143–180
Smallegange RC, Qiu YT, Van Loon JJA, Takken W (2005) Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem Senses 30:145–152
Smallegange RC, Qiu YT, Bukovinszkiné-Kiss G, Van Loon JJA, Takken W (2009) The effect of aliphatic carboxylic acids on olfaction-based host-seeking of the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol 34:933–943
Smallegange RC, Schmied WH, van Roey VNO, Spitzen J, Mukabana WR, Takken W (2010) Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar J 9:292
Smallegange RC, Bukovinszkiné-Kiss G, Otieno B, Mbadi PA, Takken W, Mukabana RW, Van Loon JJA (2012) Identification of candidate volatiles that affect the behavioural response of the malaria mosquito Anopheles gambiae sensu stricto to an active kairomone blend: laboratory and semi-field assays. Physiol Entomol 37:60–71
Takken W (2010) Push-pull strategies for vector control. Malar J 9:116
Takken W, Knols BGJ (1999) Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol 44:131–157
Vale GA, Torr, S.J. (2004) Development of bait technology to control tsetse. The Trypanosomiases, pp 509–523
Van Loon JJA, Smallegange RC, Bukovinszkiné-Kiss G, Jacobs F, De Rijk M, Mukabana RW, Verhulst NO, Menger DJ, Takken W (2015) Mosquito attraction: crucial role of carbon dioxide in formulation of a five-component blend of human-derived volatiles. J Chem Ecol 41:567–573
Verhulst NO, Andriessen R, Groenhagen U, Bukovinszkiné-Kiss G, Schulz S, Takken W, Van Loon JJA, Schraa G, Smallegange RC (2010) Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS One 5:e15829
Verhulst NO, Mukabana WR, Takken W, Smallegange RC (2011a) Human skin microbiota and their volatiles as odour baits for the malaria mosquito Anopheles gambiae s.s. Entomol Exp Appl 139:170–179
Verhulst NO, Mbadi P, Bukovinszkiné-Kiss G, Mukabana RW, Van Loon JJA, Takken W, Smallegange RC (2011b) Improvement of a synthetic lure for Anopheles gambiae using compounds produced by human skin microbiota. Malar J 10:28
Wanji S, Theodore T, Sali NA, Caroline A, Tendongfor N, Didier F (2003) Anopheles species of the Mount Cameroon region: biting habits, feeding behaviour and entomological inoculation rates. Trop Med Intl Hlth 8:643–649
WHO (1975) Manual on practical entomology in malaria: part II, methods and techniques, WHO division of malaria and other parasitic diseases. Switzerland, Geneva
Acknowledgments
We thank David Odhiambo Alila for rearing mosquitoes used for semi-field experiments. We also appreciate Andrew Brian Abuya for aspirating mosquitoes, Charles A. Oketch for working with Philemon during field experiments, and George Odhiambo Opetu for providing field meteorological data. This study was funded by a grant from the Foundation for the National Institutes of Health (FNIH) through the Grand Challenges in Global Health initiative (GCGH #121) and a sandwich PhD scholarship of Wageningen University and Research Centre, The Netherlands (CKM). CKM was hosted by icipe-TOC as a as a dissertation research internship scholar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mweresa, C.K., Mukabana, W.R., Omusula, P. et al. Enhancing Attraction of African Malaria Vectors to a Synthetic Odor Blend. J Chem Ecol 42, 508–516 (2016). https://doi.org/10.1007/s10886-016-0711-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0711-1