Abstract
Purpose
Well-designed randomized controlled trials (RCTs) are considered to represent a high level of evidence and influence medical decision-making in evidence-based medicine. When biases occur in study design, processing, and reporting of RCTs, however, it is difficult to interpret results and judge the impact of interventions. Accordingly, we evaluate the quality of RCT reporting published in the Journal of Clinical Monitoring and Computing (JCMC) using three assessment tools.
Methods
Reporting quality of RCTs published in the JCMC was evaluated through December 31, 2020, using Jadad and van Tulder scales and the Cochrane Collaboration’s risk of bias tool (CCRBT). Stepwise regression analysis was performed to identify factors associated with reporting quality.
Results
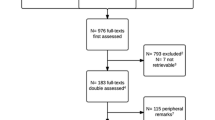
Database searches confirmed 132 RCTs in 1,507 original articles. The numbers of RCTs meeting criteria for high reporting quality were 97 (73.5%) using the Jadad scale, 99 (75.0%) using the van Tulder scale, and 19 (14.4%) with the CCRBT. Jadad scores [median score (interquartile range) = 3.0 (2.0–5.0), coefficients (95% CI) = 0.08 (0.04, 0.11), p < 0.001], van Tulder scores [median score (interquartile range) = 7.0 (5.0–8.75), coefficients (95% CI) = 0.15 (0.11, 0.20), p < 0.001], and CCRBT assessment [coefficients (95% CI) = 0.04 (0.02, 0.06), p < 0.001] increased significantly with publication year. The median score (interquartile range) of the last 5 years were 4.0 (3.0–5.0) in Jadad scores, and 8.0 (6.0–9.0) in van Tulder scores. Only 33.3% and 37.1% of articles described detailed blinding and allocation methods, respectively.
Conclusions
Reporting quality increased over time, with consistently high reporting quality in recently published JCMC RCTs.



Similar content being viewed by others
References
Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet (London England). 2017;390(10092):415–23. doi:https://doi.org/10.1016/s0140-6736(16)31592-6.
Uetani K, Nakayama T, Ikai H, Yonemoto N, Moher D. Quality of reports on randomized controlled trials conducted in Japan: evaluation of adherence to the CONSORT statement. Intern Med (Tokyo Japan). 2009;48(5):307–13. doi:https://doi.org/10.2169/internalmedicine.48.1358.
Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Krebs Seida J, Klassen TP. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ (Clinical research ed). 2009;339:b4012. doi:https://doi.org/10.1136/bmj.b4012.
Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical research ed). 2008;336(7644):601–5. doi:https://doi.org/10.1136/bmj.39465.451748.AD.
Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12. doi:https://doi.org/10.1001/jama.273.5.408.
Estellat C, Torgerson DJ, Ravaud P. How to perform a critical analysis of a randomised controlled trial. Best Pract Res Clin Rheumatol. 2009;23(2):291–303. doi:https://doi.org/10.1016/j.berh.2009.03.003.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical research ed). 2010;340:c869. doi:https://doi.org/10.1136/bmj.c869.
Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet (London England). 2001;357(9263):1191–4.
Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–9. doi:https://doi.org/10.1001/jama.276.8.637.
Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2(1):31–49. doi:https://doi.org/10.1016/0197-2456(81)90056-8.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12. doi:https://doi.org/10.1016/0197-2456(95)00134-4.
van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976). 2003;28(12):1290–9. doi:https://doi.org/10.1097/01.brs.0000065484.95996.af.
Higgins JPT, Green S. (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011.
Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–75. doi:https://doi.org/10.2522/ptj.20070147.
Kim KS, Jo JK, Chung JH, Kim JH, Choi HY, Lee SW. Quality analysis of randomized controlled trials in the International Journal of Impotence Research: quality assessment and relevant clinical impact. Int J Impot Res. 2017;29(2):65–9. doi:https://doi.org/10.1038/ijir.2016.48.
Hong SJ, Yoon DY, Cho YK, Yoon SJ, Moon JY, Baek S, Lim KJ. Characteristics and Quality of Radiologic Randomized Controlled Trials: A Bibliometric Analysis Between 1995 and 2014. AJR Am J Roentgenol. 2016;206(5):917–23. doi:https://doi.org/10.2214/ajr.15.15640.
Lee JW, Chung JH, Jo JK, Lee SW. Analysis of randomized controlled trials in Rheumatology International from 1981 to 2012: methodological assessment. Rheumatol Int. 2014;34(9):1187–93. doi:https://doi.org/10.1007/s00296-014-2963-9.
Chung JH, Kang DH, Jo JK, Lee SW. Assessing the quality of randomized controlled trials published in the Journal of Korean Medical Science from 1986 to 2011. J Korean Med Sci. 2012;27(9):973–80. doi:https://doi.org/10.3346/jkms.2012.27.9.973.
Cho HJ, Chung JH, Jo JK, Kang DH, Cho JM, Yoo TK, Lee SW. Assessments of the quality of randomized controlled trials published in International Journal of Urology from 1994 to 2011. Int J urology: official J Japanese Urol Association. 2013;20(12):1212–9. doi:https://doi.org/10.1111/iju.12150.
Blinding and allocation concealment. Sealed Envelope Ltd. (2019) https://www.sealedenvelope.com/randomisation/blinding/. Accessed 30 July 2019.
Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet (London England). 2002;359(9306):614–8. doi:https://doi.org/10.1016/s0140-6736(02)07750-4.
Lee JW, Chung JH, Jo JK, Lee SW. Assessing the quality of randomized controlled trials published in neurourology and urodynamics from 1993 to 2012. Neurourol Urodyn. 2014;33(5):472–4. doi:https://doi.org/10.1002/nau.22457.
Bridoux V, Moutel G, Roman H, Kianifard B, Michot F, Herve C, Tuech JJ. Methodological and ethical quality of randomized controlled clinical trials in gastrointestinal surgery. J Gastrointest surgery: official J Soc Surg Aliment Tract. 2012;16(9):1758–67. doi:https://doi.org/10.1007/s11605-012-1952-0.
Soares HP, Daniels S, Kumar A, Clarke M, Scott C, Swann S, Djulbegovic B. Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ (Clinical research ed). 2004;328(7430):22–4. doi:https://doi.org/10.1136/bmj.328.7430.22.
Acknowledgements
Since this manuscript is about the performance of this journal, it has been evaluated by an independent Editor from a different journal.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study conception and design: Kyu Nam Kim. Data collection: Kyu Nam Kim, Jeong Min Sung, Ji Yoon Kim and Bo Seok Kwon. Data analysis: Kyu Nam Kim, Jeong Min Sung, Ji Yoon Kim, Bo Seok Kwon. Writing the draft of the manuscript: Kyu Nam Kim and Jeong Min Sung. Critical revision of the manuscript: Kyu Nam Kim, Jeong Min Sung, Ji Yoon Kim and Bo Seok Kwon. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sung, J.M., Kim, J.Y., Kwon, B.S. et al. Risk of bias for randomized controlled trials in Journal of Clinical Monitoring and Computing. J Clin Monit Comput 37, 103–111 (2023). https://doi.org/10.1007/s10877-022-00864-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00864-8