Abstract
To investigate the usability of the SedLine® monitor in anaesthetized pigs. Five juvenile healthy pigs underwent balanced isoflurane-based general anaesthesia for surgical placement of a subcutaneous jugular venous port. The SedLine® was applied to continuously monitor electroencephalographic (EEG) activity and its modulation during anaesthesia. Computer tomography and magnetic resonance were performed to investigate the relationship between electrodes’ positioning and anatomical structures. The pediatric SedLine® EEG-sensor could be easily applied and SedLine®-generated variables collected. An EEG Density Spectral Array (DS) was displayed over the whole procedure. During surgery, the EEG signal was dominated by elevated power in the delta range (0.5–4 Hz), with an underlying broadband signal (where power decreased with increasing frequency). The emergence period was marked by a decrease in delta power, and a more evenly distributed power over the 4–40 Hz frequency range. From incision to end of surgery, mean SedLine®-generated values (± standard deviation) were overall stable [23.0 (± 2.8) Patient State Index (PSI), 1.0% (± 3.8%) Suppression Ratio (SR), 8.8 Hz (± 2.5 Hz) Spectral Edge Frequency 95% (SEF) left, 7.7 Hz (± 2.4 Hz) SEF right], quickly changing during emergence [75.3 (± 11.1) PSI, 0.0 (± 0.0) SR, 12.5 (± 6.6) SEF left 10.4 (± 6.6) SEF right]. Based on the imaging performed, the sensor does not record EEG signals from the same brain areas as in humans. SedLine®-DSA and -generated variables seemed to reflect variations in depth of anaesthesia in pigs. Further studies are needed to investigate this correlation, as well as to define the species-specific brain structures monitored by the EEG-sensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The porcine model is extensively used in translational medicine due to the anatomical and physiological similarities between humans and pigs [1,2,3,4]. The latest European Union’s (EU) Report on animal research estimated around 80,000 pigs used per year in the EU [5]. Despite the large number of animals involved in translational studies, objective and efficacious methods to assure suitable depth of anaesthesia (DoA) during experimental procedures are still missing. Ensuring an adequate DoA during surgical or diagnostic procedures is of paramount importance from an ethical perspective, as well as to guarantee scientific quality of collected data [6].
In order to assess DoA, physiological variables (e.g. heart rate, respiratory rate, blood pressure) as well as the presence of motor reactions to nociceptive stimulation, are generally evaluated. However, these parameters correlate more closely to modulation of the spinal cord than to depression of consciousness, which originates in the forebrain [7,8,9].
In order to specifically characterise and quantify brain activity during anaesthesia, several electroencephalographic (EEG) monitor devices have been developed over the last 30 years [10, 11]. Due to the complexity of raw EEG interpretation, algorithms have been patented to provide an immediate and continuous DoA index for specific use in humans. Although some of them have been used to assess DoA in pigs undergoing anaesthesia [12,13,14], none of them has been validated in this species so far. Moreover, their accuracy in assessing drug-induced modulation of the cerebral nervous system activity is debated, both in human and veterinary medicine. More recently, the use of the EEG spectrogram has been suggested as more appropriate for this purpose [15, 16]. Observation of the real-time spectrogram to facilitate interpretation of raw EEG signals in patients undergoing general anaesthesia could possibly contribute to improve DoA assessment in various animal species.
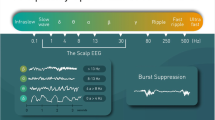
The SedLine® monitor (Masimo Corp., CA, USA; see Fig. 1) displays a real-time EEG spectrogram (Density Spectral Array [DSA]) recorded from bilateral (left and right) frontal and pre-frontal (in human) transcutaneous electrodes (RD SedLine® EEG-sensor). The time course of the raw EEG is displayed and can be exported to files in the European Data Format (.edf). Moreover, five further SedLine®-generated variables are also displayed and their time course can be exported as.csv file; these are:
-
(1)
The patient state index (PSI)—processed EEG variable related to the effect of anaesthetic agents in humans, ranging between 0 (complete EEG suppression) and 100 (fully awake). Suggested target during general anaesthesia in humans is a value between 25 and 50;
-
(2)
The suppression ratio (SR, in %)—a measure of the EEG activity suppression (isoelectric signal);
-
(3)
The left and right 95% spectral edge frequencies (SEF)—a measure of the frequency below which 95% of the total EEG power is located;
-
(4)
The electromyographic activity (EMG; in %)—a measure of muscular activity contaminating the EEG signal;
-
(5)
The artifact (ARTF, in %)—a measure of physiological (not brain-related) and environmental noise contaminating the EEG signal.
Exact algorithms used to process these SedLine®-generated variables have not been made public.
To date, the use of the SedLine® monitor has not been reported in pigs. The present pilot study aimed at investigating the usability of the SedLine® monitor in anaesthetized juvenile pigs and reporting the adequacy of the SedLine® EEG-sensor for use in these patients.
2 Materials and methods
A permission to perform the study was obtained from the Committee for Animal Experiments of the Canton of Bern (protocol number BE116/19).
Five healthy pigs (four males, one female; phenotypic Edelschwein), 10.4 ± 0.5 weeks old, with a mean body weight of 25.8 ± 2 kg were included. All animals underwent general anaesthesia for surgical placement of a subcutaneous jugular venous port. This was a preliminary step for a further experimental study, which is not reported here.
The animals were collected from the farm of origin the day before surgery and transported to a dedicated ward. They were housed in single boxes, but direct visual, olfactory and auditory contact with group mates was always granted. Pigs were fasted at least 6 h before surgery, while water was available ad libitum.
The day of surgery, clinical examination was performed and each pig was brought to the operation theatre where it was allowed to rest for at least 30 min in a quiet area. Ketamine (Narketan 10, Vetoquinol AG, Switzerland) 10 mg/kg, dexmedetomidine (Dexdomitor, Provet AG, Switzerland) 0.02 mg/kg and methadone (Methadon Streuli, Streuli Pharma AG, Switzerland) 0.2 mg/kg were mixed in the same syringe and injected intramuscularly in the neck. If after 15 min the sedation level was considered insufficient, a further dose of ketamine and/or dexmedetomidine and/or midazolam (Dormicum, Roche Pharma, Switzerland) was administered, as deemed necessary by the anaesthetist in charge. As soon as the animal showed signs of sedation, pre-oxygenation (4 L/min, 100% oxygen, via face mask) was initiated. A venous catheter was inserted in an auricular vein, and induction of anaesthesia performed with propofol (Propofol 1% MCT, Fresenius Kabi AG, Switzerland), administered intravenously (IV) to effect. The trachea was intubated and the endotracheal tube connected to a rebreathing system. Maintenance of anaesthesia was performed using isoflurane (Attane Isoflurane, Provet AG, Switzerland) in oxygen. Lack of palpebral reflex, jaw tone, absence of autonomic reactions and absence of reaction to external stimuli were targeted to ensure an adequate anaesthetic depth. An infusion of dexmedetomidine (0.004–0.008 mg/kg/h) was administered until full recovery. An arterial catheter was placed in either the coccygeal or the auricular artery. Continuous monitoring of heart rate, respiratory rate, invasive blood pressure, end-tidal carbon dioxide (ETCO2) and isoflurane (EtIso), oxygen saturation (SpO2), tidal volume and lung compliance was performed.
After full instrumentation, the area between the frontal and the occipital bone was clipped, cleaned with a warm antiseptic soap solution and shaved with a razor. Once dried, the skin was rubbed with abrasive paper (Red Dot Trace Prep, 3 M Health Care, Canada) and benzinum medicinale (Benzinum Medicinale, Hänseler AG, Switzerland) was applied using a soaked cotton ball to defatten the skin.
Once the skin was dry, the RD SedLine® EEG-sensor was positioned (adult or paediatric size). The electrodes (L2, L1, R1, R2, from left to right; see Fig. 2) were placed on a transverse line over the frontal bone, keeping their rostral border on an imaginary line running between the lateral canthi of the eyes. Left and right electrodes were placed with best possible symmetry apart the mid-sagittal line. The central CB (ground) and the caudal CT (reference) electrodes were placed on the mid-sagittal line. The adult sensor was placed first and replaced by the pediatric if judged too large (L2 and R2 too far from the temporal region, caudal to the lateral canthus of the eye). The sensor was also repositioned if the impedance was above 10 kOhm. The SedLine®-display parameters were set at 10 μV/mm and 30 mm/sec. Digital recording of the SedLine®-generated variables and raw EEG data was set. Raw EEG data were later analysed in Matlab® in order to generate the spectrogram off-line.
Positioning of the paediatric SedLine® electrodes in a pig. The electrodes line (L2, L1, R1, R2, from left to right) was placed over the frontal bone, between the eyes, keeping the rostral border of the electrodes on an imaginary line running between the lateral canthi of the eyes. The central GB (ground) and the caudal CT (reference) electrodes were placed on the mid-sagittal line
Four additional EEG electrodes (not related to the SedLine® monitor) were placed more caudally. Left (L-) and right (R-) electrodes were placed symmetrically apart the mid-sagittal line, on the same sagittal line than L1 and R1, just rostral to the caudal margin of the occipital bone (L-pos, R-pos), and in the middle between these and the RD SedLine® EEG-sensor (L-mid, R-mid, see Fig. 3). These electrodes were used to collect EEG data for another investigation, which will not be reported here.
a Volume rendering, 3D image of a pig head, representing the positioning of the SedLine® paediatric-sensor electrodes (R2, R1, L1, L2, CB = ground, CT = reference), and further four EEG electrodes (left middle = L-mid; right middle = R-mid; left posterior = L-pos; right posterior = R-pos) positioned at an equidistance between the R1/L1 and the protuberantia occipitalis. b Transverse 8 mm maximum intensity projection (MIP) image demonstrating the position of the “R1” and “L1” electrodes in relation to the zygomatic processes of the frontal bone (asterisks). The “CB” electrode is positioned on the mid sagittal aspect of the skull just dorsal to the sagittal fissure (+). ZA Zygomatic arch, M mandible. Right side is on the left side of the image. c Transverse 8 mm MIP image demonstrating the position of the “R2” and “L2” electrodes in relation to the zygomatic arch (ZA) and temporal muscle (T). Both, the “R-mid” and “L-mid” electrodes are in the region of the parietal bone. The CB electrode is similarly positioned on the mid sagittal aspect of the skull just dorsal to the sagittal fissure (+). M mandible. Right side is on the left side of the image. d Transverse 8 mm MIP image demonstrating the position of the “R-pos” and “L-pos” electrodes in relation to the protuberantia occipitalis (dagger). MH mandible head. Right side is on the left side of the image. e Sagittal image demonstrating the position of “L1”, just rostral to the left frontoparietal suture (fp), “L-mid”, dorsal to the parietal bone, and “L-pos”, dorsal to the protuberantia occipitalis (dagger). A atlas, O orbital region. Rostral is on the left side of the image. Dashed lines indicate the region of the transverse images (Fig. 3b, c, d). f Sagittal image demonstrating the position of “R1”, just rostral to the left frontoparietal suture (fp), “R-mid”, dorsal to the parietal bone, and “R-pos”, dorsal to the protuberantia occipitalis (dagger). A atlas, O orbital region. Rostral is on the left side of the image. Dashed lines indicate the region of the transverse images (Fig. 3b, c, d)
At least 10 min before starting the procedure, ropivacaine (ROPIvacain Fresenius, Fresenius Kabi, AG, Switzerland; 1 mg/kg) was injected subcutaneously at the incision site by the surgeon. Before incision, amoxicillin and clavulanic acid (Clamoxyl, GlaxoSmithKline, Switzerland; 20 mg/kg) was administered intravenously. The jugular venous catheter was placed in the left jugular vein and the subcutaneous port sutured cranial to the scapula, 2–4 cm lateral from the midline. After surgery, flunixin meglumine (Fluniximin, Graeub, Switzerland; 4.4 mg/kg) was injected intravenously. After tracheal extubation, data collection was stopped and all the monitoring tools were removed. The animal was placed in sternal recumbency in a recovery box with supplemental oxygen (4 L/min, 100%, via face mask). Sedation was administered if signs of excitation were noticed (dexmedetomidine 0.001–0.002 mg/kg IV). Once fully awake, the animal was transported back to the ward.
Two of the pigs were euthanized few days later at the end of the main study (as foreseen by the ethical authorization) and were used to perform a computed tomography and magnetic resonance imaging (MRI) of the skull, respectively. During the CT, the pediatric SedLine® sensor was left positioned as during the experiment. During the MRI, the electrodes (which are not MRI compatible) were replaced by a gadolinium-based contrast agent at the same location.
3 Results
Anaesthesia was uneventful in all animals, and no further medications were administered, except one ketamine bolus (1 mg/kg IV) during the surgical procedure in the fifth pig. Surgery time was 64 ± 8.6 min (mean ± standard deviation), time from end of surgery until sternal recumbency 75 ± 23 min, end-tidal isoflurane concentration during the procedure 1.50 ± 0.15% (approximately 0.75 of the minimum alveolar concentration for pigs[17]).
In all the animals, the adult RD SedLine® EEG-sensor was judged too large, while the pediatric size was deemed more appropriate and used for all the recordings. The impedance reading was satisfactory in all animals and none of the electrodes required replacement. The sensor remained in place without the need of further fixation (bandage, glue).
The complete raw EEG (.edf) data could be retrieved for all the animals, except for pig 2, for which only a partial recording could be retrieved. The files containing SedLine®-generated variables (.csv file) revealed brief punctual loss of data, and for one pig (pig 2) the entire file was empty. No apparent reason was found for this loss of data; no artefact, electrodes or cables disconnection were noticed.
During surgery, the EEG signal was dominated by elevated power in the delta range (0.5–4 Hz), with an underlying broadband signal (where power decreased with increasing frequency). The emergence period was marked by a decrease in delta power, and a more evenly distributed power over the 4–40 Hz frequency range (Fig. 4). The EEG spectrogram (DSA) was displayed over the whole procedure for every pig. EEG power for each frequency band (Delta 0.5–4 Hz, Theta 4–8 Hz, Alpha 8–12 Hz, Beta 12–20 Hz, High Beta 20–35 Hz) at different time points is reported in Table 1.
Spectrogram (top section) and power spectra of the electroencephalogram (EEG) for selected time points (bottom section) of pig 1 while undergoing general anaesthesia. EEG was taken from electrode R1, with the reference placed at CT (see Fig. 3a). The mean spectra present the power at each frequency at four different time points: A = 10 min before incision, B = 5 min after incision; C = 5 min before end of surgery; D = 15 min after the end of surgery. In case of presence of noise in the raw EEG data, data were taken from the closest noise-free EEG periods within a 3 min epoch. These time points are also shown on the spectrogram with vertical black lines and letters above, signalling the start and end time-points used to create the mean spectra
Sedline values were stable over the whole surgical period (from incision to end of surgery); mean values (± standard deviation) were: 23.0 (± 2.8) for PSI, 1.0% (± 3.8%) for SR, 8.8 Hz (± 2.5 Hz) for SEF l, 7.7 Hz (± 2.4 Hz) for SEF r, 3.7% (± 2.7%) for EMG, and 3.1% (± 9.6%) for ARTF. During recovery, a rapid change of the Sedline-generated variables was noticed. Values at different time points are reported in Table 2. A group level spectrogram (from 4 pigs) showing 45 min of the maintenance anesthesia period, with time periods aligned to 5 min following incision for surgery (t = 0 min) is also shown for clarity (Fig. 5).
Group level (pig 2 missing because only a partial recording could be retrieved) mean spectrogram of the maintenance anaesthesia period, with time periods aligned to 5 min following incision for surgery (t = 0 min). EEG was taken from electrode R1, with the reference placed at CT (see Fig. 3a)
During electrical cautery, artifacts were visible on the monitor, and neither a clear spectrogram/raw EEG nor derived values were available in the majority of the cases.
No skin damage was visible at the sites of electrodes application after recovery.
The computed tomography and MRI studies identified the anatomical regions underlying the electrodes (Figs. 3a–g, 6); details are reported in Table 3.
Volume rendering reconstruction of the left side of brain after skull stripping, viewed from rostral and dorsal. The round areas demonstrate the approximate brain surface coverage of the electrodes in relation with the region of the pre-frontal cortex (PFC). Black arrows: interhemispheric fissure; white arrows: olfactory bulbs
4 Discussion
The SedLine® EEG monitor was an easily applicable tool to record EEG signals in anaesthetised pigs.
Animals of 2–3 months of age were chosen, as pigs of this age are widely used in translational research due to the ease of handling. The pediatric RD SedLine® EEG-sensor appeared to fit very well to the pigs used in the present study. Sensor selection and placement may not be adequate for animals of significantly larger size. Importantly, the two sensor sizes process the EEG signal with different algorithms. As EEG dynamic modulation from general anaesthesia has been reported to differ between adult and children in humans [18], SedLine® uses a pediatric-specific signal processing engine to improve performance of the PSI. However, the exact differences in the algorithms are proprietary and not published, precluding conclusion on the impact of the sensor used.
During the procedure, single electrodes impedance remained adequate and there was no need for replacement. From author's experience with the use of RD SedLine® EEG-sensor in pigs (data not reported here), incomplete shaving is sufficient to impair signal quality, even when the skin is further prepared with soap and abrasive paper. This is in contrast with what reported by Drewnowska et al. [19], which used the RD SedLine® EEG-sensor in horses without previously shaving the hair, but applying tape bandaging to maintain a good contact over time.
Changes in the SedLine® EEG feed-speed and amplitude resolution on the display affects the data recording (Dincklage et al. [20]). Specifically, changes made to the feed-speed on the display during the recording cause a modification of the EEG sampling rate without notification. Moreover, selection of a low display resolution (e.g. 50–100 µV/mm), leads to an increased amount of zero-lines; on the other hand, a too fine resolution (1–2 µV/mm) leads to signal clipping. Therefore, it is fundamental to set the appropriate feed and resolution before starting the recording, in order to guarantee complete data acquisition if the raw EEG signal has to be assessed afterwards. In the present study, 30 mm/sec feed and 10 μV/mm amplitude were deemed appropriate.
Recordings of the numerical trends of the SedLine®-generated variables (.cvs file) was not always complete. In particular, data from the second pig were entirely missing. A human error cannot be fully excluded (i.e. involuntary change in the machine setting) but seems unlikely as the same setting was applied for further recordings in another pig on the same day and the data were correctly stored. Similar hiccup was experienced by the authors with another SedLine® device (not reported here). Thus, we suggest to record also manually the displayed values of the SedLine®-generated variables when subsequent data analysis is foreseen.
Some small gaps in data recording were present, possibly related to external interferences (e.g. cautery usage). However, these events were not precisely recorded and this potential correlation cannot be confirmed. In any case, due to the short duration of these gaps, the overall data collection quality was not compromised (Fig. 7a, b).
a PSI value for each pig (except for pig 2) is reported over time. The black vertical line shows the incision time, the other vertical lines show the end of surgery for the respective pig (see colors in the legend). The PSI values are zero when no data were recorded by the SedLine® monitor. The grey area represents the PSI range (25–50) deemed as corresponding to an adequate plane of anaesthesia in human medicine. The recovery phase in pig 5 is missing due to early removal of the EEG-sensor. b PSI, SR, 95% spectral edge left and right (SEF l and SEF r), EMG and artifact, recorded during the anaesthesia period in pig 1. The vertical black line represents the surgical incision, the red vertical line the end of surgery
No data are available yet to suggest for each SedLine®-pediatric-generated variables which target is indicative of an adequate DoA in pigs. In human medicine, a PSI between 25 and 50 is considered an optimal hypnotic state during general anaesthesia [21, 22]. In our pigs, the PSI value was always below 50 and often below 30, when clinical evaluation of DoA was deemed adequate. During the recovery phase, PSI increased quickly (mean ± SD: 75.3 ± 11.1, fifteen minutes after end of anaesthesia) reflecting the clinical picture. However, further studies are needed to investigate if SedLine®-generated variables correlate with DoA in pigs.
The report of the anatomical structures covered by the electrodes is a first step toward a better understanding of the correlation between EEG signal, skull landmarks and brain areas. Since no data are present in pigs, our results initiate refinement and standardization of electrodes positioning in this species. In humans, the SedLine® electrodes cover the regions defined as Fp1–Fp2 (prefrontal) and F7–F8 (frontal) in the 10–20 system of EEG electrodes placement [23]. Based on the computed tomography and MRI studies presented, the electrode placement applied here in pigs does not cover the same brain areas. How much these differences affect the interpretation of the EEG and the SedLine®-derived variables is not known. Precise and homogeneous information on the functional areas in the pig’s brain is still missing [25,26,27,28,29,30]. Further anatomical studies on a larger sample of animals are required.
In humans, anteriorization of the alpha waves occurs with deepening of the anaesthetic level [15, 16], justifying the relevance of monitoring EEG activity from the frontal brain lobes. No evidence of this phenomenon has been provided in pigs so far. The computed tomography and MRI studies suggest that the L1 and R1 electrodes, as placed in the present study, monitor rather the mid-frontal area, and that electrodes should potentially be placed more rostrally (on a line between the center of the eyes) to investigate EEG activity from most frontal brain areas. However, placing the sensor in a more rostral position would mean to possibly record over the frontal sinus, which is air filled. Moreover, the rigidity of the SedLine® sensors and the anatomy of the pig’s skull, would make this positioning really difficult in pigs of small size.
This paper reports the usage of the SedLine® monitor in pigs undergoing general anaesthesia. With our preparation and settings, a stable EEG signal, spectrogram and derived data were shown during the entire procedure. Artefacts can influence data recording, and data saving errors from the monitor can occur. Further studies are needed to investigate the correlation between Sedline®-generated variables and actual depth of anaesthesia in pigs.
Data availability
Not applicable.
Code availability
Not applicable.
References
Komiya K, Sato Y, Wainai T, Murayama T, Yamada M, Hiruta A, Seo N, Yoshino H, Tanaka H, Kobayashi E. Evaluation of intraoperative infusion solution using a complete anhepatic model in baby pigs. Transplant Proc. 2005;37:2341–6. https://doi.org/10.1016/j.transproceed.2005.03.104.
Bassols A, Costa C, Eckersall PD, Osada J, Sabrià J, Tibau J. The pig as an animal model for human pathologies: aproteomics perspective. Proteomics Clin Appl. 2014;8:715–31. https://doi.org/10.1002/prca.201300099.
Dawson HD, Chen C, Gaynor B, Shao J, Urban JF. The porcine translational research database: a manually curated, genomics and proteomics-based research resource. BMC Genomics. 2017. https://doi.org/10.1186/s12864-017-4009-7.
Nichols JE, La Francesca S, Niles JA, Vega SP, Argueta LB, Frank L, Christiani DC, Pyles RB, Himes BE, Zhang R, et al. Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aao3926.
European Commission Seventh Report on the Statistics on the Number of Animals used for Experimental and other Scientific Purposes in the Member States of the European Union. 2013, https://doi.org/10.1017/CBO9781107415324.004
Bradbury AG, Eddleston M, Clutton RE. Pain management in pigs undergoing experimental surgery; a literature review (2012–4). Br J Anaesth. 2016;116:37–45. https://doi.org/10.1093/bja/aev301.
Lichtner G, Auksztulewicz R, Velten H, Mavrodis D, Scheel M, Blankenburg F, von Dincklage F. Nociceptive activation in spinal cord and brain persists during deep general anaesthesia. Br J Anaesth. 2018;121:291–302. https://doi.org/10.1016/j.bja.2018.03.031.
Antognini JF. The relationship among brain, spinal cord and anesthetic requirements. Med Hypotheses. 1997;48:83–7. https://doi.org/10.1016/S0306-9877(97)90028-1.
Antognini JF, Schwartz MD. Exaggerated anesthetic requirements in the preferentially anaesthetized brain. Anesthesiology. 1993;79:1244–9.
Fahy BG, Chau DF. The technology of processed electroencephalogram monitoring devices for assessment of depth of anesthesia. Anesth Analg. 2018;126:111–7. https://doi.org/10.1213/ANE.0000000000002331.
Kaiser HA, Hight D, Avidan MS. A narrative review of electroencephalogram-based monitoring during cardiovascular surgery. Curr Opin Anaesthesiol. 2020;33:92–100. https://doi.org/10.1097/ACO.0000000000000819.
Haga HA, Tevik A, Moerch H. Bispectral index as an indicator of anaesthetic depth during isoflurane anaesthesia in the pig. J Vet Anaesth. 1999;26:3–7.
Martín-Cancho MF, Carrasco-Juménez MS, Lima JR, Ezquerra LJ, Crisóstomo V, Usón-Gargallo J. Assessment of the relationship of bispectral index values, hemodynamic changes, and recovery times associated with sevoflurane or propofol anesthesia in pigs. Am J Vet Res. 2004;65:409–16. https://doi.org/10.2460/ajvr.2004.65.409.
Llonch P, Andaluz A, Rodríguez P, Dalmau A, Jensen EW, Manteca X, Velarde A. Assessment of consciousness during propofol anaesthesia in pigs. Vet Rec. 2011. https://doi.org/10.1136/vr.d5643.
Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists: part I: background and basic signatures. Anesthesiology. 2015;123:937–60. https://doi.org/10.1097/ALN.0000000000000841.
Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KFK, Salazar-Gomez AF, Harrell PG, Sampson AL, Cimenser A, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci USA. 2013. https://doi.org/10.1073/pnas.1221180110.
Eger EI 2nd, Johnson BH, Weiskopf RB, Holmes MA, Yasuda N, Targ A, Rampil IJ. Minimum alveolar concentration of I-653 and isoflurane in pigs: definition of a supramaximal stimulus. Anesth Analg. 1988;67:1174–6.
Akeju O, Pavone KJ, Thum JA, Firth PG, Westover MB, Puglia M, Shank ES, Brown EN, Purdon PL. Age-dependency of sevoflurane-induced electroencephalogram dynamics in children. Br J Anaesth. 2015;115:i66-76. https://doi.org/10.1093/bja/aev114.
Drewnowska O, Turek B, Lisowska B, Short CE. Preliminary study of the use of root with sedline® eeg monitoring for assessment of anesthesia depth in 6 horses. Appl Sci. 2020. https://doi.org/10.3390/app10031050.
von Dincklage F, Jurth C, Schneider G, García PS, Kreuzer M. Technical considerations when using the EEG export of the SEDLine root device. J Clin Monit Comput. 2021. https://doi.org/10.1007/s10877-020-00578-9.
Lee KH, Kim YH, Sung YJ, Oh MK. The patient state index is well balanced for propofol sedation. Hippokratia. 2015;19:235–8.
Ortega III, H.R. PSI 25–50 range for optimal hypnotic state for general anesthesia, 2007. https://www-qa2.masimo.de/siteassets/uk/documents/pdf/psi_25-50_white_paper.pdf.
Homan RW, Herman J, Purdy P. Cerebral location of international 10-20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987; 66(4):376–82. https://doi.org/10.1016/0013-4694(87)90206-9.
Jelsing J, Hay-Schmidt A, Dyrby T, Hemmingsen R, Uylings HBM, Pakkenberg B. The prefrontal cortex in the Göttingen minipig brain defined by neural projection criteria and cytoarchitecture. Brain Res Bull. 2006;70:322–36. https://doi.org/10.1016/j.brainresbull.2006.06.009.
Bjarkam CR, Glud AN, Orlowski D, Sørensen JCH, Palomero-Gallagher N. The telencephalon of the Göttingen minipig, cytoarchitecture and cortical surface anatomy. Brain Struct Funct. 2017;222:2093–114. https://doi.org/10.1007/s00429-016-1327-5.
Sauleau P, Lapouble E, Val-Laillet D, Malbert CH. The pig model in brain imaging and neurosurgery. Animal. 2009;3:1138–51. https://doi.org/10.1017/S1751731109004649.
Rausova P, Vochozkova P, Vidinska D, Hrnciarova E, Bohuslavova B, Macakova M, Valekova I, Juhas S, Macakova M, Valekova I, et al. Porcine model of Huntington’s disease porcine disease. London: IntechOpen; 2017.
Watanabe H, Andersen F, Simonsen CZ, Evans SM, Gjedde A, Cumming P. MR-based statistical atlas of the Göttingen minipig brain. Neuroimage. 2001;14:1089–96. https://doi.org/10.1006/nimg.2001.0910.
Schmidt V. Comparative anatomy of the pig brain—An integrative magnetic resonance imaging (MRI) study of the porcine brain with special emphasis on the external morphology of the cerebral cortex, vol. 183. Germany: VVB Laufersweiler Verlag; 2015.
Clouard C, Meunier-Salaün MC, Meurice P, Malbert CH, Val-Laillet D. Combined compared to dissociated oral and intestinal sucrose stimuli induce different brain hedonic processes. Front Psychol. 2014;5:1–12. https://doi.org/10.3389/fpsyg.2014.00861.
Funding
Open access funding provided by University of Bern. This study was supported by the Swiss National Science Foundation (Spark) (Grant No. n°CRSK-3_190926/1).
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by AM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Permission to perform the study has been obtained from the Committee for Animal Experiments of the Canton of Bern; protocol number BE116/19.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mirra, A., Casoni, D., Barge, P. et al. Usability of the SedLine® electroencephalographic monitor of depth of anaesthesia in pigs: a pilot study. J Clin Monit Comput 36, 1635–1646 (2022). https://doi.org/10.1007/s10877-022-00807-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00807-3