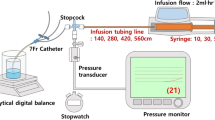
Abstract
Time lags between the initiation of a continuous drug infusion and achievement of a steady state delivery rate present an important safety concern. At least 3 factors contribute to these time lags: (1) dead volume size, (2) the ratio between total system flow and dead volume, and (3) startup delay. While clinicians employ both peristaltic pumps and syringe pumps to propel infusions, there has been no head-to-head comparison of drug delivery between commercially available infusion pumps with these distinct propulsion mechanisms. We quantified the delivery of a model drug by peristaltic and syringe pumps at clinically relevant flow rates using spectrophotometric absorbance. Delivery curves were modeled and compared, and the time required to reach 5% (T5), 50% (T50), and 95% (T95) of the intended delivery rate was reported. The ability to overcome the combined effects of startup delay and dead volume differed between syringe and peristaltic pumps. T5, T50, and T95 were shorter for the peristaltic pump at higher flow rates. T50 and T95 were shorter for the syringe pump at lower flow rates. The ability to overcome the effects of dead volume was overall similar between the syringe and peristaltic pumps, as was the response to consecutive changes in drug infusion rates. Startup delay and dead volume in carrier-based infusion systems cause substantial time lags to reaching intended delivery rates. Peristaltic and syringe pumps are similarly susceptible to dead volume effects. Startup performance differed between peristaltic and syringe pumps; their relative performance may be dependent on flow rate.




Similar content being viewed by others
Data availability
Any raw data presented in this manuscript is available by written request to the corresponding author.
Code availability
Not applicable.
Change history
06 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10877-021-00801-1
Abbreviations
- CI:
-
Confidence interval,
- FDA:
-
Food and Drug Administration
- MB:
-
Methylene blue
- MFP:
-
Multivariable fractional polynomial
References
Bartels K, Moss DR, Peterfreund RA. An analysis of drug delivery dynamics via a pediatric central venous infusion system: quantification of delays in achieving intended doses. Anesth Analg. 2009;109:1156–61.
Moss DR, Bartels K, Peterfreund GL, Lovich MA, Sims NM, Peterfreund RA. An in vitro analysis of central venous drug delivery by continuous infusion: the effect of manifold design and port selection. Anesth Analg. 2009;109:1524–9.
Lovich MA, Doles J, Peterfreund RA. The impact of carrier flow rate and infusion set dead-volume on the dynamics of intravenous drug delivery. Anesth Analg. 2005;100:1048–55.
Lovich MA, Peterfreund GL, Sims NM, Peterfreund RA. Central venous catheter infusions: a laboratory model shows large differences in drug delivery dynamics related to catheter dead volume. Crit Care Med. 2007;35:2792–8.
Lovich MA, Peterfreund RA. Drug flow through clinical infusion systems: how modeling of the common-volume helps explain clinical events. Pharm Technol Hosp Pharm. 2017;2:49–61.
Neff T, Fischer J, Fehr S, Baenziger O, Weiss M. Start-up delays of infusion syringe pumps. Paediatr Anaesth. 2001;11:561–5.
Mandel JE. Understanding infusion pumps. Anesth Analg. 2018;126:1186–9.
An J, Butterfield RD, Sims NM. Evaluation of clinical infusion pump performance through downstream microdrop monitoring: a preliminary study. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC). 2020:6066–9.
U.S. Food and Drug Administration. Syringe Pump problems with fluid flow continuity at low infusion rates can result in serious clinical consequences: FDA safety communication. 2016. https://www.fdanews.com/ext/resources/files/2016/08/08-25-16-pumpsafetynotice.pdf?1480880246#:~:text=The%20FDA%20is%20informing%20health,can%20result%20in%20serious%20clinical. Accessed September 24, 2020.
Butterfield RD. Personal e-email communication. 2020.
Baeckert M, Batliner M, Grass B, Buehler PK, Daners MS, Meboldt M, Weiss M. Performance of modern syringe infusion pump assemblies at low infusion rates in the perioperative setting. Br J Anaesth. 2020;124:173–82.
Center for Devices and Radiological Health, U.S. Food and Drug Administration. White paper: infusion pump improvement initiative. 2020. https://www.fda.gov/medical-devices/infusion-pumps/white-paper-infusion-pump-improvement-initiative. Accessed September 24, 2020.
Tsao AC, Lovich MA, Parker MJ, Zheng H, Peterfreund RA. Delivery interaction between co-infused medications: an in vitro modeling study of microinfusion. Paediatr Anaesth. 2013;23:33–9.
Parker MJ, Lovich MA, Tsao AC, Deng H, Houle T, Peterfreund RA. Novel pump control technology accelerates drug delivery onset in a model of pediatric drug infusion. Anesth Analg. 2017;124:1129–34.
Parker MJ, Lovich MA, Tsao AC, Wei AE, Wakim MG, Maslov MY, Tsukada H, Peterfreund RA. Computer control of drug delivery by continuous intravenous infusion: bridging the gap between intended and actual drug delivery. Anesthesiology. 2015;122:647–58.
User manual: sapphire multi-therapy and dedicated infusion pumps. 2019. https://www.qcore.com/sapphire-user-manuals. Accessed October 22, 2020.
Medfusion syringe pump model 3500 operator’s manual, software version 6. 2013. http://www.medfusionpump.com/assets/literature/manuals/Operators_Manual_3500_40-6370-01A.pdf. Accessed October 22, 2020.
Funding
This study was funded by a Development Grant from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital.
Author information
Authors and Affiliations
Contributions
LEG: this author designed the experiments, performed data analysis, and wrote and reviewed the manuscript. AK: this author performed data collection, data analysis, and reviewed the manuscript. DA: this author designed the experiments and reviewed the manuscript. HD: this author performed statistical analyses and wrote and reviewed the manuscript. NMS: this author designed the experiments and wrote and reviewed the manuscript. RAP: this author designed the experiments, performed data analysis, and wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Robert A. Peterfreund and Nathaniel M. Sims are co-inventors on US Patents 9,764,087 and 10,758,672, and European Patent EP-2575933 for infusion pump control technology.
Ethical approval
Waived for benchtop research not involving human or animal subjects.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gibson, L.E., Knudsen, A.S., Arney, D. et al. Lag times to steady state drug delivery by continuous intravenous infusion: direct comparison of peristaltic and syringe pump performance identifies contributions from infusion system dead volume and pump startup characteristics. J Clin Monit Comput 36, 1489–1498 (2022). https://doi.org/10.1007/s10877-021-00790-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00790-1