Abstract
Purpose
Altered gravity environments introduce cardiovascular changes that may require continuous hemodynamic monitoring in both spaceflight and terrestrial analogs. Conditions in such environments are often prohibitive to direct/invasive methods and therefore, indirect measurement techniques must be used. This study compares two common cardiac measurement techniques used in the human spaceflight domain, pulse contour analysis (PCA—Nexfin) and inert gas rebreathing (IGR—Innocor), in subjects completing ergometer exercise under altered gravity conditions simulated using a tilt paradigm.
Methods
Seven subjects were tilted to three different angles representing Martian, Lunar, and microgravity conditions in the rostrocaudal direction. They completed a 36-min submaximal cardiovascular exercise protocol in each condition. Hemodynamics were continuously monitored using Nexfin and Innocor.
Results
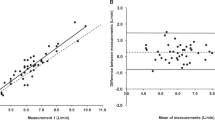
Linear mixed-effects models revealed a significant bias of \(25.7\pm 1.7\) ml (\(p<0.0001\)) in stroke volume and \(3.19\pm 0.18\) l/min (\(p<0.0001\)) in cardiac output, with Nexfin measuring greater than Innocor in both variables. These values are in agreement with a Bland-Altman analysis. The correlation of stroke volume and cardiac output measurements between Nexfin and Innocor were \(r=0.54\) (\(p<0.0001\)) and \(r=0.76\) (\(p<0.0001\)) respectively.
Conclusion
There is a poor agreement in absolute stroke volume and cardiac output values between measurement via PCA (Nexfin) and IGR (Innocor) in subjects who are exercising in simulated altered gravity environments. These results suggest that the chosen measurement method and device greatly impacts absolute measurements of cardiac output. However, there is a good level of agreement between the two devices when measuring relative changes. Either of these devices seem adequate to capture cardiac changes, but should not be solely relied upon for accurate measurement of absolute cardiac output.






Similar content being viewed by others
Data availability
The datasets analyzed for this study are publicly available, a repository can be found on GitHub: https://github.com/rswhittle/altered-gravity-exercise.
References
Buckey JC. Space physiology. Oxford: Oxford University Press; 2006.
Norsk P, Asmar A, Damgaard M, Christensen NJ. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J Physiol. 2015;593(3):573–84. https://doi.org/10.1113/jphysiol.2014.284869.
Fritsch-Yelle JM, Charles JB, Jones MM, Wood ML. Microgravity decreases heart rate and arterial pressure in humans. J Appl Physiol. 1996;80(3):910–4. https://doi.org/10.1152/jappl.1996.80.3.910.
Foldager N, Andersen TA, Jessen FB, Ellegaard P, Stadeager C, Videbæk R, Norsk P. Central venous pressure in humans during microgravity. J Appl Physiol. 1996;81(1):408–12. https://doi.org/10.1152/jappl.1996.81.1.408.
Buckey JC, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW, Meyer DM, Blomqvist CG. Central venous pressure in space. J Appl Physiol. 1996;81(1):19–25. https://doi.org/10.1152/jappl.1996.81.1.19.
MacNamara JP, Dias KA, Sarma S, Lee SM, Martin D, Romeijn M, Zaha VG, Levine BD. Cardiac effects of repeated weightlessness during extreme duration swimming compared with spaceflight. Circulation. 2021;143(15):1533–5. https://doi.org/10.1161/circulationaha.120.050418.
Diedrich A, Paranjape SY, Robertson D. Plasma and blood volume in space. Am J Med Sci. 2007;334(1):80–6. https://doi.org/10.1097/MAJ.0b013e318065b89b
Zhang LF. Invited Review: Vascular adaptation to microgravity: what have we learned? J Appl Physiol. 2001;91:2415–30.
Eckberg DL, Halliwill JR, Beightol LA, Brown TE, Taylor JA, Goble R. Human vagal baroreflex mechanisms in space. J Physiol. 2010;588(7):1129–38. https://doi.org/10.1113/jphysiol.2009.186650.
Platts SH, Martin DS, Stenger MB, Perez SA, Ribeiro LC, Summers R, Meck JV. Cardiovascular adaptations to long-duration head-down bed rest. Aviation Space Environ Med. 2009;80(5):A29-36. https://doi.org/10.3357/asem.br03.2009.
Petersen LG, Damgaard M, Petersen JCG, Norsk P. Mechanisms of increase in cardiac output during acute weightlessness in humans. J Appl Physiol. 2011;111(2):407–11. https://doi.org/10.1152/japplphysiol.01188.2010.
Geerts BF, Aarts LP, Jansen JR. Methods in pharmacology: measurement of cardiac output. Brit J Clin Pharmacol. 2011;71(3):316–30. https://doi.org/10.1111/j.1365-2125.2010.03798.x.
Di Rienzo M, Castiglioni P, Iellamo F, Volterrani M, Pagani M, Mancia G, Karemaker JM, Parati G. Dynamic adaptation of cardiac baroreflex sensitivity to prolonged exposure to microgravity: data from a 16-day spaceflight. J Appl Physiol. 2008;105(5):1569–75. https://doi.org/10.1152/JAPPLPHYSIOL.90625.2008.
Verheyden B, Liu J, Beckers F, Aubert AE. Adaptation of heart rate and blood pressure to short and long duration space missions. Respir Physiol Neurobiol. 2009;169(SUPPL.):S13–6. https://doi.org/10.1016/J.RESP.2009.03.008.
Hughson RL, Shoemaker JK, Blaber AP, Arbeille P, Greaves DK, Pereira-Junior PP, Xu D. Cardiovascular regulation during long-duration spaceflights to the International Space Station. J Appl Physiol. 2012;112(5):719–27. https://doi.org/10.1152/japplphysiol.01196.2011.
Norsk P, Damgaard M, Petersen L, Gybel M, Pump B, Gabrielsen A, Christensen NJ. Vasorelaxation in space. Hypertension. 2006;47(1):69–73. https://doi.org/10.1161/01.HYP.0000194332.98674.57.
Limper U, Gauger P, Beck LE. Upright cardiac output measurements in the transition to weightlessness during parabolic flights. Aviation Space Environ Med. 2011;82(4):448–54. https://doi.org/10.3357/ASEM.2883.2011.
Bougharouat N, Clemensen P. Photoacoustic Gas Analyser for RMS II (Respiratory Monitoring System). In Proceedings of the sixth European symposium on life sciences research in space, Trondheim. 1996. p. 321–5.
Ameloot K, Van De Vijver K, Broch O, Van Regenmortel N, De Laet I, Schoonheydt K, Dits H, Bein B, Malbrain ML. Nexfin noninvasive continuous hemodynamic monitoring: validation against continuous pulse contour and intermittent transpulmonary thermodilution derived cardiac output in critically ill patients. Sci World J. 2013;2013:519080. https://doi.org/10.1155/2013/519080.
Trinkmann F, Sampels M, Doesch C, Papavassiliu T, Brade J, Schmid-Bindert G, Hoffmann U, Borggrefe M, Kaden JJ, Saur J. Is arterial pulse contour analysis using Nexfin a new option in the noninvasive measurement of cardiac output? A pilot study. J Cardiothoracic Vasc Anesthesia. 2013;27(2):283–7. https://doi.org/10.1053/j.jvca.2012.08.011.
Monnet X, Picard F, Lidzborski E, Mesnil M, Duranteau J, Richard C, Teboul JL. The estimation of cardiac output by the Nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care. 2012;16(5):R212. https://doi.org/10.1186/cc11846.
Bartels SA, Stok WJ, Bezemer R, Boksem RJ, Van Goudoever J, Cherpanath TG, Van Lieshout JJ, Westerhof BE, Karemaker JM, Ince C. Noninvasive cardiac output monitoring during exercise testing: Nexfin pulse contour analysis compared to an inert gas rebreathing method and respired gas analysis. J Clin Monit Comput. 2011;25(5):315–21. https://doi.org/10.1007/s10877-011-9310-4.
Middlemiss JE, Cocks A, Paapstel K, Maki-Petaja KM, Sunita Wilkinson IB, McEniery CM. Evaluation of inert gas rebreathing for determination of cardiac output: influence of age, gender and body size. Hypertens Res. 2019;42(6):834–44. https://doi.org/10.1038/s41440-018-0179-1.
Peyton PJ, Bailey M, Thompson BR. Reproducibility of cardiac output measurement by the nitrous oxide rebreathing technique. J Clin Monit Comput. 2009;23(4):233–6. https://doi.org/10.1007/s10877-009-9187-7.
Corte TJ, Wells AU, Gatzoulis MA, Cramer D, Ward S, Macdonald PS, Dimopoulos K, Wort SJ. Non-invasive assessment of pulmonary blood flow using an inert gas rebreathing device in fibrotic lung disease. Thorax. 2010;65(4):341–5. https://doi.org/10.1136/thx.2009.121129.
Jakovljevic DG, Nunan D, Donovan G, Hodges LD, Sandercock GR, Brodie DA. Comparison of cardiac output determined by different rebreathing methods at rest and at peak exercise. Eur J Appl Physiol. 2008;102(5):593–9. https://doi.org/10.1007/s00421-007-0631-4.
Fontana P, Boutellier U, Toigo M. Reliability of measurements with Innocor(TM) during exercise. Int J Sports Med. 2009;30(10):747–53. https://doi.org/10.1055/s-0029-1225340.
Stok WJ, Baisch F, Hillebrecht A, Schulz H, Meyer M, Karemaker JM. Noninvasive cardiac output measurement by arterial pulse analysis compared with inert gas rebreathing. J Appl Physiol. 1993;74(6):2687–93. https://doi.org/10.1152/jappl.1993.74.6.2687.
Grensemann J. Cardiac output monitoring by pulse contour analysis, the technical basics of less-invasive techniques. Front Med. 2018;5(Mar):64. https://doi.org/10.3389/fmed.2018.00064.
Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74(5):2566–73. https://doi.org/10.1152/jappl.1993.74.5.2566.
Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47(2):131–41. https://doi.org/10.1007/s11517-008-0359-2.
Bogert LWJ, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90(4):437–46. https://doi.org/10.1113/expphysiol.2005.030262.
Truijen J, Van Lieshout JJ, Wesselink WA, Westerhof BE. Noninvasive continuous hemodynamic monitoring. J Clin Monit Comput. 2012;26(4):267–78. https://doi.org/10.1007/s10877-012-9375-8.
Perel A, Settels JJ. Totally non-invasive continuous cardiac output measurement with the Nexfin CO-Trek. In: Vincent JL, editor. Annual update in intensive care and emergency medicine 2011. Heidelberg: Springer; 2011. p. 434–42. https://doi.org/10.1007/978-3-642-18081-1.
Diaz-Artiles A, Heldt T, Young LR. Short-term cardiovascular response to short-radius centrifugation with and without ergometer exercise. Front Physiol. 2018;9:1492. https://doi.org/10.3389/fphys.2018.01492.
Ayotte B, Seymour J, McIlroy MB. A new method for measurement of cardiac output with nitrous oxide. J Appl Physiol. 1970;28(6):863–6. https://doi.org/10.1152/jappl.1970.28.6.863.
Laszlo G. Respiratory measurements of cardiac output: from elegant idea to useful test. J Appl Physiol. 2004;96(2):428–37. https://doi.org/10.1152/japplphysiol.01074.2001.
Innovision ApS. Innocor: Inert Gas Rebreathing Method, COR-MAN-0000-004-IN. Tech. Rep., Innovision ApS, Glamsbjerg, Denmark. 2013. http://innocc.dk/wp-content/uploads/2014/06/Innocor-Inert-Gas-Rebreathing-Method-A-9.pdf.
Gabrielsen A, Videbæk R, Schou M, Damgaard M, Kastrup J, Norsk P. Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin Sci. 2002;102(2):247–52. https://doi.org/10.1042/cs1020247.
Fisher D, Fisher E, Budd J, Rosen S, Goldman M. The incidence of patent foramen ovale in 1,000 consecutive patients. A contrast transesophageal echocardiography study. Chest. 1995;107(6):1504–9. https://doi.org/10.1378/CHEST.107.6.1504.
Diaz-Artiles A, Navarro Tichell P, Perez F. Cardiopulmonary responses to sub-maximal ergometer exercise in a hypo-gravity analog using head-down tilt and head-up tilt. Front Physiol. 2019;10:720. https://doi.org/10.3389/fphys.2019.00720.
Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B Stat Methodol. 2011;73(1):3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x.
Wood SN. Generalized Additive Models: An Introduction with R. 2nd ed. Boca Raton: Taylor & Francis Group; 2017. https://doi.org/10.1201/9781315370279.
Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. https://doi.org/10.1177/096228029900800204.
Zou G. Confidence interval estimation for the Bland–Altman limits of agreement with multiple observations per individual. Stat Methods Med Res. 2011;22(6):630–42. https://doi.org/10.1177/0962280211402548.
Bartlett MS. Properties of sufficiency and statistical tests. Proc R Soc Lond Ser A Math Phys Sci. 1937;160(901):268–82. https://doi.org/10.1098/rspa.1937.0109.
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. nlme: linear and Nonlinear Mixed Effects Model. R Package Version 3. 2020.
King TS, Chinchilli VM, Carrasco JL. A repeated measures concordance correlation coefficient. Stat Med. 2007;26(16):3095–113. https://doi.org/10.1002/SIM.2778.
Carrasco JL, King TS, Chinchilli VM. The concordance correlation coefficient for repeated measures estimated by variance components. J Biopharm Stat. 2009;19(1):90–105. https://doi.org/10.1080/10543400802527890.
Whittle RS, Diaz-Artiles A. Modeling individual differences in cardiovascular response to gravitational stress using a sensitivity analysis. J Appl Physiol. 2021;130:1983–2001. https://doi.org/10.1152/japplphysiol.00727.2020.
Sakka S, Kozieras J, Thuemer O, van Hout N. Measurement of cardiac output: a comparison between transpulmonary thermodilution and uncalibrated pulse contour analysis. Brit J Anaesthesia. 2007;99(3):337–42. https://doi.org/10.1093/bja/aem177.
Buhre W, Weyland A, Kazmaier S, Hanekop GG, Baryalei MM, Sydow M, Sonntag H. Comparison of cardiac output assessed by pulse-contour analysis and thermodilution in patients undergoing minimally invasive direct coronary artery bypass grafting. J Cardiothoracic and Vasc Anesthesia. 1999;13(4):437–40. https://doi.org/10.1016/S1053-0770(99)90216-1.
Pittman J, Bar-Yosef S, SumPing J, Sherwood M, Mark J. Continuous cardiac output monitoring with pulse contour analysis: a comparison with lithium indicator dilution cardiac output measurement. Crit Care Med. 2005;33(9):2015–21. https://doi.org/10.1097/01.CCM.0000179021.36805.1F.
Felbinger TW, Reuter DA, Eltzschig HK, Bayerlein J, Goetz AE. Cardiac index measurements during rapid preload changes: a comparison of pulmonary artery thermodilution with arterial pulse contour analysis. J Clin Anesthesia. 2005;17(4):241–8. https://doi.org/10.1016/j.jclinane.2004.06.013.
Bein B, Worthmann F, Tonner PH, Paris A, Steinfath M, Hedderich J, Scholz J. Comparison of esophageal Doppler, pulse contour analysis, and real-time pulmonary artery thermodilution for the continuous measurement of cardiac output. J Cardiothoracic and Vasc Anesthesia. 2004;18(2):185–9. https://doi.org/10.1053/j.jvca.2004.01.025.
Bogert L, Wesseling K, Schraa O, Van Lieshout E, de Mol B, van Goudoever J, Westerhof B, van Lieshout J. Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia. 2010;65(11):1119–25. https://doi.org/10.1111/J.1365-2044.2010.06511.X.
Broch O, Renner J, Gruenewald M, Meybohm P, Schöttler J, Caliebe A, Steinfath M, Malbrain M, Bein B. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012;67(4):377–83. https://doi.org/10.1111/J.1365-2044.2011.07018.X.
van der Spoel A, Voogel A, Folkers A, Boer C, Bouwman R. Comparison of noninvasive continuous arterial waveform analysis (Nexfin) with transthoracic Doppler echocardiography for monitoring of cardiac output. J Clin Anesthesia. 2012;24(4):304–9. https://doi.org/10.1016/J.JCLINANE.2011.09.008.
Bubenek-Turconi S, Craciun M, Miclea I, Perel A. Noninvasive continuous cardiac output by the Nexfin before and after preload-modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesthesia Analgesia. 2013;117(2):366–72. https://doi.org/10.1213/ANE.0B013E31829562C3.
Critchley LAH, Huang L, Zhang J. Continuous cardiac output monitoring: what do validation studies tell us? Curr Anesthesiol Rep. 2014;4(3):242–50. https://doi.org/10.1007/S40140-014-0062-9.
Manen O, Dussault C, Sauvet F, Montmerle-Borgdorff S. Limitations of stroke volume estimation by non-invasive blood pressure monitoring in hypergravity. PLoS ONE. 2015;10(3):e0121936. https://doi.org/10.1371/JOURNAL.PONE.0121936.
Goswami N, Bruner M, Xu D, Bareille MP, Beck A, Hinghofer-Szalkay H, Blaber AP. Short-arm human centrifugation with 0.4 g at eye and 0.75 g at heart level provides similar cerebrovascular and cardiovascular responses to standing. Eur J Appl Physiol. 2015a;115(7):1569–75. https://doi.org/10.1007/S00421-015-3142-8.
Goswami N, Evans J, Schneider S, Mvd Wiesche, Mulder E, Rössler A, Hinghofer-Szalkay H, Blaber AP. Effects of individualized centrifugation training on orthostatic tolerance in men and women. PLoS ONE. 2015b;10(5):e0125780. https://doi.org/10.1371/JOURNAL.PONE.0125780.
Verma AK, Xu D, Bruner M, Garg A, Goswami N, Blaber AP, Tavakolian K. Comparison of autonomic control of blood pressure during standing and artificial gravity induced via short-arm human centrifuge. Front Physiol. 2018;9(6):712. https://doi.org/10.3389/FPHYS.2018.00712.
Harms MPM, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci. 1999;97(3):291–301. https://doi.org/10.1042/CS0970291.
Shibata S, Levine BD. Biological aortic age derived from the arterial pressure waveform. J Appl Physiol (Bethesda, Md, 1985). 2011;110(4):981–7. https://doi.org/10.1152/JAPPLPHYSIOL.01261.2010.
Chen G, Meng L, Alexander B, Tran N, Kain Z, Cannesson M. Comparison of noninvasive cardiac output measurements using the Nexfin monitoring device and the esophageal Doppler. J Clin Anesthesia. 2012;24(4):275–83. https://doi.org/10.1016/J.JCLINANE.2011.08.014.
Arai T, Limper U, Gauger P, Beck L. Pulse contour methods to estimate cardiovascular indices in micro- and hypergravity. Aviation Space Environ Med. 2013;84(11):1178–85. https://doi.org/10.3357/ASEM.3683.2013.
Peyton PJ, Thompson B. Agreement of an inert gas rebreathing device with thermodilution and the direct oxygen fick method in measurement of pulmonary blood flow. J Clin Monit Comput. 2004;18(5–6):373–8. https://doi.org/10.1007/s10877-005-1589-6.
Siebenmann C, Rasmussen P, Sørensen H, Zaar M, Hvidtfeldt M, Pichon A, Secher NH, Lundby C. Cardiac output during exercise: a comparison of four methods. Scand J Med Sci Sports. 2015;25(1):e20–7. https://doi.org/10.1111/sms.12201.
Saur J, Fluechter S, Trinkmann F, Papavassiliu T, Schoenberg S, Weissmann J, Haghi D, Borggrefe M, Kaden JJ. Noninvasive determination of cardiac output by the inert-gas-rebreathing method—comparison with cardiovascular magnetic resonance imaging. Cardiology. 2009;114(4):247–54. https://doi.org/10.1159/000232407.
Sperlich B, Haegele M, Krüger M, Schiffer T, Holmberg HC, Mester J. Cardio-respiratory and metabolic responses to different levels of compression during submaximal exercise. Phlebology. 2011;26(3):102–6. https://doi.org/10.1258/PHLEB.2010.010017.
Prochnau D, Forberg T, Kühnert H, Heinke M, Figulla HR, Surber R. Optimization of the atrioventricular delay during cardiac resynchronization therapy using a device for non-invasive measurement of cardiac index at rest and during exercise. EP Europace. 2012;14(2):249–53. https://doi.org/10.1093/EUROPACE/EUR296.
Wiegand G, Binder W, Ulmer H, Kaulitz R, Riethmueller J, Hofbeck M. Noninvasive cardiac output measurement at rest and during exercise in pediatric patients after interventional or surgical atrial septal defect closure. Pediatr Cardiol. 2012;33(7):1109–14. https://doi.org/10.1007/S00246-012-0239-2.
Fortney S, Vroman N, Beckett W, Permutt S, LaFrance N. Effect of exercise hemoconcentration and hyperosmolality on exercise responses. J Appl Physiol (Bethesda, MD, 1985). 1988;65(2):519–24. https://doi.org/10.1152/JAPPL.1988.65.2.519.
Sowton E, Bloomfield D, Jones N, Higgs B, Campbell E. Recirculation time during exercise. Cardiovasc Res. 1968;2(4):341–5. https://doi.org/10.1093/CVR/2.4.341.
Hughes WE, Casey DP. Aortic wave reflection during orthostatic challenges: influence of body position and venous pooling. Am J Hypertens. 2017. https://doi.org/10.1093/ajh/hpw138
Hughson RL, Peterson SD, Yee NJ, Greaves DK. Cardiac output by pulse contour analysis does not match the increase measured by rebreathing during human spaceflight. J Appl Physiol. 2017;123(5):1145. https://doi.org/10.1152/JAPPLPHYSIOL.00651.2017.
Acknowledgements
Figure 2 created with BioRender.com.
Funding
This work was supported by the National Aeronautics and Space Administration (NASA) Human Research Program (HRP), Grants 80NSSC19K0020 and 80NSSC20K1521.
Author information
Authors and Affiliations
Contributions
RSW and AD-A contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RSW, LM, LGP, and AD-A. The first draft of the manuscript was written by RSW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Human Research Protection Program of Texas A&M University with Institutional Review Board number IRB2018-1338D.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Whittle, R.S., Stapleton, L.M., Petersen, L.G. et al. Indirect measurement of absolute cardiac output during exercise in simulated altered gravity is highly dependent on the method. J Clin Monit Comput 36, 1355–1366 (2022). https://doi.org/10.1007/s10877-021-00769-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00769-y