Abstract
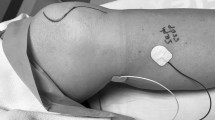
The demand for intraoperative monitoring (IOM) of lumbar spine surgeries has escalated to accommodate more challenging surgical approaches to prevent perioperative neurologic deficits. Identifying impending injury of individual lumbar roots can be done by assessing free-running EMG and by monitoring the integrity of sensory and motor fibers within the roots by eliciting somatosensory (SEP), and motor evoked potentials. However, the common nerves for eliciting lower limb SEP do not monitor the entire lumbar plexus, excluding fibers from L1 to L4 roots. We aimed to technically optimize the methodology for saphenous nerve SEP (Sap-SEP) proposed for monitoring upper lumbar roots in the operating room. In the first group, the saphenous nerve was consecutively stimulated in two different locations: proximal in the thigh and distal close to the tibia. In the second group, three different recording derivations (10–20 International system) to distal saphenous stimulation were tested. Distal stimulation yielded a higher Sap-SEP amplitude (mean ± SD) than proximal: 1.36 ± 0.9 µV versus 0.62 ± 0.6 µV, (p < 0.0001). Distal stimulation evoked either higher (73%) or similar (12%) Sap-SEP amplitude compared to proximal in most of the nerves. The recording derivation CPz–cCP showed the highest amplitude in 65% of the nerves, followed by CPz–Fz (24%). Distal stimulation for Sap-SEP has advantages over proximal stimulation, including simplicity, lack of movement and higher amplitude responses. The use of two derivations (CPz–cCP, CPz–Fz) optimizes Sap-SEP recording.





Similar content being viewed by others
References
Aminoff MJ, Eisen AA. AAEM minimonograph 19: somatosensory evoked potentials. Muscle Nerve. 1998;21:277–90. https://doi.org/10.1002/(sici)1097-4598(199803)21:3%3c277::aid-mus1%3e3.0.co;2-7.
Eccher M. Intraoperative neurophysiologic monitoring: are we really that bad? J Clin Neurophysiol. 2012;29:157–9. https://doi.org/10.1097/WNP.0b013e31824ff6d0.
Eisen A, Elleker G. Sensory nerve stimulation and evoked cerebral potentials. Neurology. 1980;30:1097–105.
Kaukoranta E, Hamalainen M, Sarvas J, Hari R. Mixed and sensory nerve stimulations activate different cytoarchitectonic areas in the human primary somatosensory cortex SI. Neuromagnetic recordings and statistical considerations. Exp Brain Res. 1986;63:60–6.
Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. Oxford: OUP; 2013.
Krishnakumar R, Srivatsa N. Multimodal intraoperative neuromonitoring in scoliosis surgery: a two-year prospective analysis in a single centre. Neurol India. 2017;65:75–9. https://doi.org/10.4103/0028-3886.198189.
Laratta JL, Ha A, Shillingford JN, Makhni MC, Lombardi JM, Thuet E, Lehman RA, Lenke LG. Neuromonitoring in spinal deformity surgery: a multimodality approach. Glob Spine J. 2018;8:68–77. https://doi.org/10.1177/2192568217706970.
MacDonald DB. Individually optimizing posterior tibial somatosensory evoked potential P37 scalp derivations for intraoperative monitoring. J Clin Neurophysiol. 2001;18:364–71.
MacDonald DB, Al Zayed Z, Stigsby B. Tibial somatosensory evoked potential intraoperative monitoring: recommendations based on signal to noise ratio analysis of popliteal fossa, optimized P37, standard P37, and P31 potentials. Clin Neurophysiol. 2005;116:1858–69. https://doi.org/10.1016/j.clinph.2005.04.018.
MacDonald DB, Dong C, Quatrale R, Sala F, Skinner S, Soto F, Szelenyi A. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin Neurophysiol. 2019;130:161–79. https://doi.org/10.1016/j.clinph.2018.10.008.
Macefield G, Burke D, Gandevia SC. The cortical distribution of muscle and cutaneous afferent projections from the human foot. Electroencephalogr Clin Neurophysiol. 1989;72:518–28.
Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976). 2019;44:369–76. https://doi.org/10.1097/BRS.0000000000002822.
Nakanishi K, Inoue K, Hadoush H, Sunagawa T, Ochi M. Dipole orientation of receptive fields in the somatosensory cortex after stimulation of the posterior tibial nerve in humans. J Clin Neurophysiol. 2014;31:236–40. https://doi.org/10.1097/WNP.0000000000000044.
Nuwer MR. Intraoperative monitoring of neural function. Amsterdam: Elsevier; 2008.
Overzet K, Kazewych M, Jahangiri FR. Multimodality intraoperative neurophysiological monitoring (IONM) in anterior hip arthroscopic repair surgeries. Cureus. 2018;10: e3346. https://doi.org/10.7759/cureus.3346.
Raynor BL, Padberg AM, Lenke LG, Bridwell KH, Riew KD, Buchowski JM, Luhmann SJ. Failure of intraoperative monitoring to detect postoperative neurologic deficits: a 25-year experience in 12,375 spinal surgeries. Spine (Phila Pa 1976). 2016;41:1387–93. https://doi.org/10.1097/BRS.0000000000001531.
Rosenfalck A. Early recognition of nerve disorders by near-nerve recording of sensory action potentials. Muscle Nerve. 1978;1:360–7. https://doi.org/10.1002/mus.880010504.
Silverstein J, Mermelstein L, DeWal H, Basra S. Saphenous nerve somatosensory evoked potentials: a novel technique to monitor the femoral nerve during transpsoas lumbar lateral interbody fusion. Spine (Phila Pa 1976). 2014;39:1254–60. https://doi.org/10.1097/BRS.0000000000000357.
Taniguchi M, Nadstawek J, Pechstein U, Schramm J. Total intravenous anesthesia for improvement of intraoperative monitoring of somatosensory evoked potentials during aneurysm surgery. Neurosurgery. 1992;31:891–7.
Thirumala P, Zhou J, Natarajan P, Balzer J, Dixon E, Okonkwo D, Hamilton DK. Perioperative neurologic complications during spinal fusion surgery: incidence and trends. Spine J. 2017;17:1611–24. https://doi.org/10.1016/j.spinee.2017.05.020.
Thuet ED, Winscher JC, Padberg AM, Bridwell KH, Lenke LG, Dobbs MB, Schootman M, Luhmann SJ. Validity and reliability of intraoperative monitoring in pediatric spinal deformity surgery: a 23-year experience of 3436 surgical cases. Spine (Phila Pa 1976). 2010;35:1880–6. https://doi.org/10.1097/BRS.0b013e3181e53434.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. This study was approved by the IRB at Mount Sinai System (IRB # 18-01277).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez Roldán, M.Á., Mora Granizo, F., Oflidis, V. et al. Optimizing the methodology for saphenous nerve somatosensory evoked potentials for monitoring upper lumbar roots and femoral nerve during lumbar spine surgery: technical note. J Clin Monit Comput 36, 1079–1085 (2022). https://doi.org/10.1007/s10877-021-00737-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00737-6