Abstract
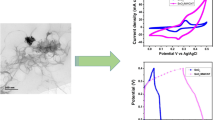
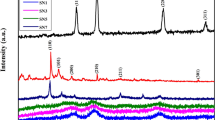
In this present work, the transition metal oxides of SnO2 and SnO2/rGO nanocomposite were synthesized through a facile hydrothermal method for supercapacitor electrode material applications. The structural, morphological, and elemental analysis of the synthesised samples were characterised by X-ray diffractometer technique (XRD), Field emission scanning electron microscopy (FESEM), Energy dispersive X-ray analysis (EDX), and High-resolution transmission electron microscopy (HR-TEM). The morphology of SnO2 was an agglomeration of quasi-spherical-shaped particles with a diameter range of 12–19 nm, as observed using the HR-TEM technique. The optical properties were characterised by UV-vis and Raman spectroscopy. The electrochemical performance of SnO2 and SnO2/rGO nanocomposite electrode was studied in a 3 M KOH electrolyte. A specific capacitance of 346 F g− 1 at a current density of 0.95 A g− 1 for the SnO2/rGO nanocomposite electrode was recorded, which was significantly higher than that of the as-synthesised SnO2 electrode (267 F g− 1). The higher capacitance obtained was due to the synergistic effect of excellent conductivity and a high surface area of rGO within the composite electrode. The exceptional electrochemical properties clearly indicate that the SnO2/rGO nanocomposites are the best for highly efficient pseudocapacitor electrodes in future energy storage device applications.











Similar content being viewed by others
Data Availability
The data that supports the findings of this study is not openly available due to ethical reasons.
References
Liu, Y.; Jiao, Y.; Zhang, Z.; Qu, F.; Umar, A.; Wu, X. Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation, smart gas sensor, and supercapacitor applications. ACS Appl. Mater. Inter. 2014, 6, 2174–2184.
Hahn, Y.-B. Metal oxide nanostructures and their applications; American Scientific Publisher: 2010.
Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854.
Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323.
Conway, B.E. Electrochemical supercapacitors: scientific fundamentals and technological applications; Springer Science & Business Media: 1999.
Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531.
He, Y.; Chen, W.; Gao, C.; Zhou, J.; Li, X.; Xie, E. An overview of carbon materials for flexible electrochemical capacitors. Nanoscale 2013, 5, 8799–8820.
Geng, J.; Ma, C.; Zhang, D.; Ning, X. Facile and fast synthesis of SnO2 quantum dots for high performance solid-state asymmetric supercapacitor. J. Alloy. Comp. 2020, 825, 153850.
Senthilkumar, V.; Kadumudi, F.B.; Ho, N.T.; Kim, J.-W.; Park, S.; Bae, J.-S.; Choi, W.M.; Cho, S.; Kim, Y.S. NiO nanoarrays of a few atoms thickness on 3D nickel network for enhanced pseudocapacitive electrode applications. J. Power Sources 2016, 303, 363–371.
He, C.; Xiao, Y.; Dong, H.; Liu, Y.; Zheng, M.; Xiao, K.; Liu, X.; Zhang, H.; Lei, B. Mosaic-structured SnO2@ C porous microspheres for high-performance supercapacitor electrode materials. Electrochim. Acta 2014, 142, 157–166.
Ding, S.; Luan, D.; Boey, F.Y.C.; Chen, J.S.; Lou, X.W.D. SnO 2 nanosheets grown on graphene sheets with enhanced lithium storage properties. Chem. Commun. 2011, 47, 7155–7157.
Selvan, R.K.; Perelshtein, I.; Perkas, N.; Gedanken, A. Synthesis of hexagonal-shaped SnO2 nanocrystals and SnO2@ C nanocomposites for electrochemical redox supercapacitors. J. Phys. Chem. C 2008, 112, 1825–1830.
Liang, K.; Cheang, T.Y.; Wen, T.; Xie, X.; Zhou, X.; Zhao, Z.W.; Shen, C.C.; Jiang, N.; Xu, A.W. Facile preparation of porous Mn2SnO4/Sn/C composite cubes as high performance anode material for lithium-ion batteries. J. Phys. Chem. C 2016, 120, 3669–3676.
Zhu, Z.; Wang, S.; Du, J.; Jin, Q.; Zhang, T.; Cheng, F.; Chen, J. Ultrasmall Sn nanoparticles embedded in nitrogen-doped porous carbon as high-performance anode for lithium-ion batteries. Nano Lett. 2014, 14, 153–157.
Rakhi, R.; Alshareef, H.N. Enhancement of the energy storage properties of supercapacitors using graphene nanosheets dispersed with metal oxide-loaded carbon nanotubes. J. Power Sources 2011, 196, 8858–8865.
Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131.
Godlaveeti, S.K.; Somala, A.R.; Sana, S.S.; Ouladsmane, M.; Ghfar, A.A.; Nagireddy, R.R. Evaluation of pH effect of tin oxide (SnO2) nanoparticles on photocatalytic degradation, dielectric and supercapacitor applications. J. Clust. Sci. 2022, 33, 1635–1644.
Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Mothudi, B.; Zorkani, I.; Jorio, A. X-ray diffraction investigations of nanostructured ZnO coated with reduced graphene oxide. In Proceedings of the Journal of Physics: Conference Series, 2019; p. 012011.
Chen, Y.-L.; Hu, Z.-A.; Chang, Y.-Q.; Wang, H.-W.; Zhang, Z.-Y.; Yang, Y.-Y.; Wu, H.-Y.J.T. Zinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrix. J.Phys.Chem 2011, 115, 2563–2571.
Ebrahimi Naghani, M.; Neghabi, M.; Zadsar, M.; Abbastabar Ahangar, H.J. Synthesis and characterization of linear/nonlinear optical properties of graphene oxide and reduced graphene oxide-based zinc oxide nanocomposite. Sci.Rep. 2023, 13, 1496.
Khan, S.; Zulfiqar; Khan, T.; Khan, R.; Khan, M.; khattak, S.A.; Khan, G. Investigation of structural, optical, electrochemical and dielectric properties of SnO2/GO nanocomposite. J. Mater. Sci.: Mater. Electron. 2019, 30, 10202–10210.
Tran, T.V.; Alsaiari, M.; Harraz, F.A.; Nabgan, W.; Nguyen, D.T.D.; Nguyen, C.V. Taguchi L9 (34) orthogonal array design for photocatalytic degradation of methylene blue dye by green ZnO particles biosynthesized by Chrysanthemum spp. Flower extract. Water 2023, 15, 2186.
Sorekine, G.; Anduwan, G.; Waimbo, M.N.; Osora, H.; Velusamy, S.; Kim, S.; Kim, Y.S.; Charles, J. Photocatalytic studies of copper oxide nanostructures for the degradation of methylene blue under visible light. J. Mol. Struct. 2022, 1248, 131487.
Kadri, L.; Abderrahmane, A.; Bulai, G.; Carlescu, A.; Doroftei, C.; Motrescu, I.; Gurlui, S.; Leontie, L.; Adnane, M. optical and structural analysis of TiO2–SiO2 nanocomposite thin films fabricated via pulsed laser deposition technique. Nanomaterials 2023, 13, 1632.
Kamble, V.B.; Umarji, A.M. Defect induced optical bandgap narrowing in undoped SnO2 nanocrystals. AIP Adv. 2013, 3, 082120.
Scott, J. Raman spectrum of SnO2. J. Chem. Phys. 1970, 53, 852–853.
Traylor, J.G.; Smith, H.; Nicklow, R.; Wilkinson, M. Lattice dynamics of rutile. Phys. Rev. B 1971, 3, 3457.
Peercy, P.; Morosin, B. Pressure and temperature dependences of the Raman-active phonons in Sn O 2. Phys. Rev. B 1973, 7, 2779.
Gharbi, O.; Tran, M.T.; Tribollet, B.; Turmine, M.; Vivier, V. Revisiting cyclic voltammetry and electrochemical impedance spectroscopy analysis for capacitance measurements. Electrochim. Acta 2020, 343, 136109.
Abebe, E.M.; Ujihara, M. Simultaneous electrodeposition of ternary metal oxide nanocomposites for high-efficiency supercapacitor applications. ACS Omega 2022, 7, 17161–17174.
Wu, M.; Zhang, L.; Wang, D.; Xiao, C.; Zhang, S. Cathodic deposition and characterization of tin oxide coatings on graphite for electrochemical supercapacitors. J. Power Sources 2008, 175, 669–674.
Wee, G.; Soh, H.Z.; Cheah, Y.L.; Mhaisalkar, S.G.; Srinivasan, M. Synthesis and electrochemical properties of electrospun V 2 O 5 nanofibers as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 6720–6725.
Li, H.; Gao, Y.; Wang, C.; Yang, G. A simple electrochemical route to access amorphous mixed-metal hydroxides for supercapacitor electrode materials. Adv. Energy Mater. 2015, 5, 1401767.
Liu, M.; Li, B.; Zhou, H.; Chen, C.; Liu, Y.; Liu, T. Extraordinary rate capability achieved by a 3D “skeleton/skin” carbon aerogel–polyaniline hybrid with vertically aligned pores. Chem. Commun. 2017, 53, 2810–2813.
Senthilkumar, V.; Kim, Y.S.; Chandrasekaran, S.; Rajagopalan, B.; Kim, E.J.; Chung, J.S. Comparative supercapacitance performance of CuO nanostructures for energy storage device applications. RSC Adv. 2015, 5, 20545–20553.
Dang, Y.-Q.; Ren, S.-Z.; Liu, G.; Cai, J.; Zhang, Y.; Qiu, J. Electrochemical and capacitive properties of carbon dots/reduced graphene oxide supercapacitors. Nanomaterials 2016, 6, 212.
Geng, L.; Yan, F.; Dong, C.; An, C. Design and regulation of novel MnFe2O4@ C nanowires as high performance electrode for supercapacitor. Nanomaterials 2019, 9, 777.
Jebakumar Immanuel Edison, T.N.; Atchudan, R.; Lee, Y.R. Facile synthesis of carbon encapsulated RuO2 nanorods for supercapacitor and electrocatalytic hydrogen evolution reaction. Inter. J. Hydrog. Energy 2019, 44, 2323–2329.
Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; RanjithKumar, D.; Lee, Y.R. Direct growth of iron oxide nanoparticles filled multi-walled carbon nanotube via chemical vapour deposition method as high-performance supercapacitors. Inter. J. Hydrog. Energy 2019, 44, 2349–2360.
Saravanakumar, B.; Ravi, G.; Ganesh, V.; Ravichandran, S.; Sakunthala, A.; Yuvakkumar, R.J. Low surface energy and pH effect on SnO2 nanoparticles formation for supercapacitor applications. J. Nanosci. Nanotechnol. 2019, 19, 3429–3436.
Godlaveeti, S.K.; Somala, A.R.; Sana, S.S.; Ouladsmane, M.; Ghfar, A.A.; Nagireddy, R.R.J. Evaluation of pH effect of tin oxide (SnO2) nanoparticles on photocatalytic degradation, dielectric and supercapacitor applications. J. Clust. Sci. 2022, 33, 1635–1644.
Zhang, Y.; Liu, M.; Sun, S.; Yang, L.J. The preparation and characterization of SnO2/rGO nanocomposites electrode materials for supercapacitor. Adv. Compos. Lett. 2020, 29, 2633366X20909839.
Joshi, N.C.; Rawat, B.; Bisht, H.; Gajraj, V.; Kumar, N.; Chetana, S.; Gururani, P.J. Synthesis and supercapacitive behaviour of SnO2/r-GO nanocomposite. Synth. Met. 2022, 289, 117132.
Liu, M.; Sun, S.; Yang, L.; Yin, S.J. Ultrathin SnO2 nanorod/reduced graphene oxide nanosheet composites for electrochemical supercapacitor applications with excellent cyclic stability. Int. J. Mater. Res. 2018, 109, 743-750.311
Eedulakanti, S.R.; Gampala, A.K.; Rao, K.V.; Chakra, C.S.; Gedela, V.; Boddula, R.J. Ultrasonication assisted thermal exfoliation of graphene-tin oxide nanocomposite material for supercapacitor. Mater. Sci. Energy Technol. 2019, 2, 372–376.
Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Chandrasekaran, P.; Perumal, S.; Arunachalam, P.; Raja, P.B.; Sethuraman, M.G.; Lee, Y.R. Electrochemically exfoliated graphene sheets as electrode material for aqueous symmetric supercapacitors. Surf. Coat. Technol. 2021, 416, 127150.
Shinde, P.A.; Lokhande, V.C.; Patil, A.M.; Ji, T.; Lokhande, C.D.J. Single-step hydrothermal synthesis of WO3-MnO2 composite as an active material for all-solid-state flexible asymmetric supercapacitor. Int. J. Hydrog. Energy 2018, 43, 2869–2880.
Barclay, M.; Firestein, K.; Wang, X.; Motta, N.; Dubal, D.; Ostrikov, K.J. Plasma-activated water for improved intercalation and pseudocapacitance of MnO2 supercapacitor electrodes. Mater. Today Sustain. 2023, 22, 100388.
Acknowledgements
The authors gratefully acknowledge the postgraduate research committees of PAPUA NEW GUINEA UNIVERSITY OF TECHNONLOGY and THE UNIVERSITY OF GOROKA for their financial assistance.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Experimental, data curation, Formal analysis, and writing-original draft, H.O.; Experimental, Formal analysis, M.W.; Formal analysis, investigation and visualization, D.K. and G.A.; Conceptualization, data curation, visualization, supervision and Writing-review & editing, S.V.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors confirm that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osora, H., Kolkoma, D., Anduwan, G. et al. Hydrothermally Grown SnO2 and SnO2/rGO Nanocomposite and Its Physio-Electrochemical Studies for Pseudocapacitor Electrode Applications. J Clust Sci 35, 891–901 (2024). https://doi.org/10.1007/s10876-023-02517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02517-5