Abstract
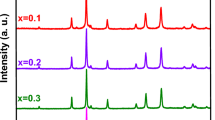
Microwave combustion method (MCM) was utilized to synthesize nanoparticles of Mn- Mn doped CuFe2O4 (Mn: CuFe2O4). The crystallite size ranged from 19 to 22 nm, determined from the XRD studies of Mn: CuFe2O4 nanoparticles employing the Debye Scherrer formula. X-ray photoelectron spectroscopy (XPS) analysis was employed to determine the chemical elements and their respective oxidation states. The agglomerative nanoparticles exhibited a spherical shape as observed through HR-SEM. The elemental composition of the nanoparticles was determined through EDX analysis, which indicated the presence of Mn, Cu, Fe, and O. The sample completely analyzed by TEM SAED, EPR and BET. The samples produced were analyzed using diffuse reflectance spectroscopy, which revealed energy gap values ranging from 2.10 to 2.30 eV. The observed photoluminescence emissions in the visible range of 415–450 nm in all samples suggest the presence of oxygen vacancies. Magnetic properties viz. remanent magnetization, coercivity, and saturation magnetization deduced from the hysteresis curves (B-H). Further nanoparticles that were synthesized were subjected to photocatalytic degradation (PCD) of rhodamine B under visible light. The spinel system of CuFe2O4 exhibits reactivity towards H2O2, leading to a process akin to the Fenton reaction. The chemical components of RhB dye confirmed by Total ion chromatography. The photocatalytic performance of the CMF5 sample is superior when compared to the other samples. The possible mechanisms amenable to the PCD method are elaborated.





















Similar content being viewed by others
References
R. Kant (2012). Textile dyeing industry an environmental hazard. Nat. Sci. 04, 22–26.
P. Nuengmatcha, S. Chanthaia, R. Mahachaia, and W. C. Oh (2016). Visible light-driven photocatalytic degradation of rhodamine B and industrial dyes (texbrite BAC-L and texbrite NFW-L) by ZnO-graphene-TiO2 composite. J. Environ. Chem. Eng. 4, 2170–2177.
N. Barka, S. Qourzal, A. Assabbane, A. Nounahb, and Y. A. Ichou (2008). Factors influencing the photocatalytic degradation of Rhodamine B by TiO2-coated non-woven paper. J. Photochem. Photobio. A: Chem. 195, 346–351.
Y. Wang, N. Lin, Y. Gong, R. Wang, and X. Zhang (2021). Cu-Fe embedded cross-linked 3D hydrogel for enhanced reductive removal of Cr(VI): Characterization, performance and mechanisms. Chemosphere 280, 130–663.
Y. Wang, Y. Gong, N. Lin, L. Yu, B. Du, and X. Zhang (2022). Enhanced removal of Cr(VI) from aqueous solution by stabilized nanoscale zero-valent iron and copper bimetal intercalated montmorillonite. J. Colloid Interface Sci. 606, 941–952.
Y. Wang, R. Wang, N. Lin, Y. Wang, and X. Zhang (2021). Highly efficient microwave-assisted Fenton degradation bisphenol A using iron oxide modified double perovskite intercalated montmorillonite composite nanomaterial as catalyst. J. Colloid Interface Sci. 594, 446–459.
Y. Wang, L. Yu, R. Wang, Y. Wang, and X. Zhang (2020). Reactivity of carbon spheres templated Ce/LaCo0.5Cu0.5O3 in the microwave induced H2O2 catalytic degradation of salicylic acid: Characterization, kinetic and mechanism studies. J. Colloid Interface Sci. 574, 74–86.
Y. Yang, W. Ji, X. Li, Z. Zheng, F. Bi, M. Yang, J. Xu, and X. Zhang (2021). Insights into the degradation mechanism of perfluorooctanoic acid under visible-light irradiation through fabricating flower-shaped Bi5O7I/ZnO n-n heterojunction microspheres. Chem. Eng. J. 420, 129934.
K. Yue, X. Zhang, S. Jiang, J. Chen, Y. Yang, F. Bi, and Y. Wang (2021). Recent advances in strategies to modify MIL-125 (Ti) and its environmental applications. J. Mol. Liq. 335, 116108.
N. Liu, J. Wang, M. Tian, J. Lei, J. Wang, W. Shi, X. Zhang, and L. Tang (2021). Boron nitride nanosheets decorated MIL-53(Fe) for efficient synergistic ibuprofen photocatalytic degradation by persulfate activation. J. Colloid Interface Sci. 603, 270–281.
K. Kaviyarasu, A. Ayeshamariam, E. Manikandan, J. Kennedy, R. Ladchumananandasivam, U. U. Gomes, M. Jayachandran, and M. Maaza (2016). Solution processing of CuSe quantum dots: photocatalytic activity under RhB for UV and visible-light solar irradiation. Mater. Sci. Eng. B. 210, 1–9.
Z. Zhang, S. Zhai, M. Wang, H. Ji, L. He, C. Ye, C. Wang, S. Fang, and H. Zhang (2016). Photocatalytic degradation of rhodamine B by using a nanocomposite of cuprous oxide, three-dimensional reduced graphene oxide, and nanochitosan prepared via one-pot synthesis. J. Alloys Compd. 659, 101–111.
K. Kaviyarasu, E. Manikandan, J. Kennedy, M. Jayachandran, R. Ladchumananandasiivam, U. U. Gomes, and M. Maaza (2016). Synthesis and characterization studies of NiO nanorods for enhancing Solar cell efficiency using photon up conversion materials. Ceram. Inter. 42, 8385–8394.
C. P. Bai, X. F. Xiong, W. Q. Gong, D. X. Feng, M. Xian, Z. X. Ge, and N. Xu (2011). Removal of rhodamine B by ozone-based advanced oxidation process. Desalination 278, 84–90.
L. Du, J. Wu, and C. Hu (2012). Electrochmical oxidation of Rhodamine B on RuO2-PdO TiO2/TiElectrode. Electrochim. Acta. 68, 69–73.
M. F. Hou, L. Liao, W. D. Zhang, X. Y. Tang, H. F. Wan, and G. C. Yin (2011). Degradation of rhodamineB by Fe(0)-based Fenton process with H2O2. Chemosphere 83, 1279–1283.
S. K. Das, P. Ghosh, I. Ghosh, and A. K. Guha (2008). Adsorption of rhodamine B on Rhizopusoryzae: Role of functional groups and cell wall components. Colloid. Surf. B. 65, 30–34.
S. Shakir, A. F. Elkafrawy, H. F. Ghoneimy, S. G. E. Beheir, and M. Refaar (2010). Removal of RhodamineB (a basic dye) and thoron (an acidic dye) from dilute aqueous solutions and wastewater stimulants by ion flotation. Water Res. 44, 1449–1461.
X. Wang, J. Wang, P. Guo, W. Guo, and C. Wang (2009). Degradation of rhodamine B in aqueous solution by using swirling jet-induced cavitation combined with H2O2. J. Hazard. Mater. 169, 486–491.
F. Y. Hung-Lin Chen, X. X. Liu, H. Jing, B. Gao, D. Zou, and C. C. Chen (2021). Visible-light-driven photocatalysis of carbon dioxide and organic pollutants by MFeO2 (M = Li, Na, or K). J. Colloid Interface Sci. 601, 758–772.
Y. Y. Lin, F. Y. Kuo-Yu Hung, Y. M. D. Liu, and J. H. Lin (2022). Chiing-Chang Chen Photocatalysts of quaternary composite, bismuth oxyfluoride/bismuth oxyiodide/ graphitic carbon nitride: Synthesis, characterization, and photocatalytic activity. Mol. Catal. 528, 112463.
A. Tony Dhiwahar, S. Maruthamuthu, R. Marnadu, M. Sundararajan, M. Aslam Manthrammel, P. Mohd Shkir, V. R. Sakthivel, and M. Reddy (2021). Improved photocatalytic degradation of rhodamine B under visible light and magnetic properties using microwave combustion grown Ni doped copper ferrite spinel nanoparticles. Solid State Sci. 113, 106542.
A. T. Dhiwahar, S. Maruthamuthu, K. V. Chandekar, M. S. Hamdy, M. Shkir, M. Sundararajan, and P. Sakthivel (2021). Facile synthesis of Cu1- xCoxFe2O4 (0 ≤ x ≤ 0.5) nanoparticles with enhanced magnetic and photocatalytic performances for organic dye degradation. Adv. Powder. Technol. 32 (11), 4030–4041.
C. Y. Toe, Z. Zheng, H. Wu, J. Scott, R. Amal, and Y. H. Ng (2018). Photocorrosion of cuprous oxide in hydrogen production: Rationalising self-oxidation or self reduction, Angew. Chem. Int. 57, 13613–13617.
H. Wu, X. Y. Kong, X. Wen, S. P. Chai, E. C. Lovell, J. Tangand, and Y. H. Ng (2021). Metal-organic framework decorated cuprous oxide nanowires for long lived charges applied in selective photocatalytic CO2 reduction to CH4. Angew. Chem. Int. 60, 8455–8459.
H. Wu, Z. Zheng, C. Y. Toe, X. Wen, J. N. Hart, R. Amal, and Y. H. Ng (2020). A pulse electrodeposited amorphous tunnel layer stabilises Cu2O for efficient photoelectrochemical water splitting under visible light irradiation. J. Mater. Chem. A. 8, 5638–5646.
X. Wang, A. Wang, and J. Ma (2017). Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard Mater. 336, 81–92.
W. Chen, Y. Zhou, J. Lu, X. Huang, W. Wu, C. Lin, and Q. Wang (2016). Effects of Li+substitution on the structural and magnetic properties of Co0.5Mn0.5Fe2O4 particles. Ceram. Inter. 42, 1114–1121.
Y. Koseoglu, F. Alan, M. Tan, R. Yilgin, and M. Ozturk (2012). Low temperature hydrothermal synthesis and characterization of Mn doped cobalt ferrite nanoparticles. Ceram. Inter. 38, 3625–3634.
Z. Deligoz, A. Baykal, E. E. Tanrıverdi, Z. Durmus, and M. S. Toprak (2012). Synthesis, structural and electrical properties of triethylene glycol (TREG)stabilized Mn0.2Co0.8Fe2O4 NPs. Mater. Res. Bulletin. 47, 537–543.
G. Raja, S. Gopinath, and K. Sivakumar (2016). Synthesis of spinel nanocrystalline ZnFe2O4: Structural, optical, magnetic and electrical properties. Ceram. Int. 42, 8763–8768.
M. Sajjia, M. Oubaha, T. Prescott, and A. G. Olabi (2010). Development of cobalt ferrite powder preparation employing the sol–gel technique and its structural characterization. J. Alloys Comp. 506, 400–406.
A. R. Denton and N. W. Ashcroft (1991). Vegard’s law. Phys. Rev. A. 43, 3161–3164.
M. Shkir, K. V. Chandekar, T. Alshahrani, A. Kumar, and S. AlFaify (2020). A novel terbium doping effect on physical properties of lead sulfide nanostructures: A facile synthesis and characterization. J. Mater. Res. 35, 1–12.
M. Sundararajan and L. John Kennedy (2017). Photocatalytic removal of rhodamine B under Irradiationof visible light using Co1-xCuxFe2O4 (0 ≤ x ≤ 0.5) nanoparticles. J. Environ. Chem. Eng. 5, 4075–4092.
X. Liang, P. Liu, H. He, G. Wei, T. Chen, W. Tan, F. Tan, J. Zhu, and R. Zhu (2016). The variation of cationic microstructure in Mn-doped spinel ferrite during calcination and its effect formaldehyde catalytic oxidation. J. Hazard. Mater. 306, 305–312.
E. R. Kumar, R. Jayaprakash, G. S. Devi, and P. S. PrasadaReddy (2014). Magnetic, dielectric and sensing properties of manganese substituted copper ferrite nanoparticles. J. Magn. Magn. Mater. 355, 87–92.
L. K. Wu, H. Wu, H. B. Zhang, H. Z. Cao, G. Y. Hou, Y. P. Tang, and G. Q. Zheng (2018). Graphene oxide/CuFe2O4 foam as an efficient absorbent for arsenic removal fromWater. Chem. Eng. J. 334, 1808–1819.
F. Y. Hung-Lin Chen, X. X. Liu, Y. Y. Lin, H. Jing, G. Y. Liu, B. Gao, D. Zou, and C. C. Chen (2022). Photoreduction of carbon dioxide and photodegradation of organic pollutants using alkali cobalt oxides MCoO2 (M = Li or Na) as catalysts. J. Environ. Manag. 313, 114930.
M. A. Rehman, I. Yusoff, and Y. Alias (2015). Fluoride adsorption by doped and un-doped magnetic ferrites CuCexFe2-xO4: Preparation, characterization, optimization and modeling for effectual remediation technologies. J. Hazard. Mater. 299, 316–324.
M. Parthibavarman, V. Hariharan, and C. Sekar (2011). High-sensitivity humidity sensor based on SnO2nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C. 31, 840–844.
V. Manikandan, M. Singh, B. C. Yadav, and J. C. Denardin (2018). Fabrication of lithium substituted copper ferrite (Li- CuFe2O4) thin film as an efficient gas sensor at room temperature. J. Sci. Adv. Mater. Dev. 3 (2), 145–150.
N. Subhashini, S. Revathi, M. Ubaidullah, A. M. Al-Enizi, S. Muthulakshmi, D. Thiripurasundari, S. F. Shaikh, A. Nafady, M. M. Abdulhameed, N. B. Alanzi, R. I. Alkhalifah, C. S. Dash, M. Sundararajan, and M. Sukumar (2023). Gd3+ substituted BiFeO3 perovskite nanoparticles: facile synthesis, characterization, and applications in heterogeneous catalysis. Dalton Trans. 52, 2735.
K. Jahanara and S. Farhadi (2019). A magnetically separable plate-like cadmium titanate–copper ferrite nanocomposite with enhanced visible-light photocatalytic degradation performance for organic contaminants. RSC Adv. 9, 15615.
P. Kubelka and F. Munk (1931). EinBeitragzurOptik der farbanstriche. Z. Tech. Phys. 12, 593601.
M. T. Hammad, J. K. Salem, A. A. Amsha, and N. K. Hejazy (2018). Optical and magneticcharacterizations of zinc substituted copper ferrite synthesized by a co-precipitation chemical method. J. Alloys Compd. 741, 123–130.
G. T. Anand and L. J. Kennedy (2013). One-pot microwave combustion synthesis of porous Zn1-xCu xAl2O4 (0 ≤ x ≤ 0.5) Spinel Nanostructures. J. Nanosci. Nanotech. 13, 1–8.
S. K. Jesudoss, J. J. Vijaya, L. J. Kennedy, P. I. Rajan, H. A. Al-Lohedan, R. J. Ramalingam, K. Kaviyarasu, and M. Bououdina (2016). Studies on the efficient dual performanceof Mn1–xNixFe2O4 spinel, nanoparticles in photodegradation and antibacterial activity. J. Photochem. Photobiol. B: Biol. 165, 121–132.
Y. Zhou, Y. Wang, T. Wen, S. Zhang, B. Chang, Y. Guo, and B. Yang (2016). Mesoporous Cd1xZnxSmicrospheres with tunable band gap and high specific surface areas for enhanced visible- ligh-driven hydrogen generation. J. Colloid. Interface Sci. 467, 97–104.
C. Murugan, R. A. Nataraj, M. P. Kumar, S. Ravichandran, and A. Pandikumar (2019). Enhance charge transfer process of bismuthvanadate interleaved graphitic carbon nitride nanohybrids in mediator-free direct Z Scheme photoelectrocatalytic water splitting. Energy Techn. Environ. Sci. 4, 4653–4663.
K. Kombaiah, J. J. Vijaya, L. J. Kennedy, M. Bououdina, and B. A. Najar (2018). Conventional and microwave combustion synthesis of optomagnetic CuFe2O4 nanoparticles for hyperthermia studies. J. Phys. Chem. Solids. 115, 162–171.
C. Qin, Z. Li, G. Chen, Y. Zhao, and T. Lin (2015). Fabrication and visible-light photocatalytic behaviorof perovskite praseodymium ferrite porous nanotubes. J. Power Sources. 285, 178–184.
A. Aslam, M. Z. Abid, K. Rafiq, A. Rauf, and E. Hussain (2023). Tunable sulphur doping on CuFe2O4 nanostructures for the selective elimination of organic dyes from water. Sci. Rep. 13 (1), 6306.
E. Hema, B. R. Venkatraman. Enhanced photo-catalytic activity of TiO2 supported Mn-codoped CuFe2O4 magnetically recyclable nano-composites.
T. S. Natarajan, M. Thomas, K. Natarajan, H. C. Bajaj, and R. J. Tayade (2011). Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 169, 126–134.
Acknowledgement
The authors (Jothi Ramalingam R) acknowledge the financial support through Researchers Supporting Project number (RSP2023R354) King Saud University, Riyadh 11451, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhiwahar, A.T., Revathi, S., Basith, N.M. et al. Investigation of Visible Light Driven Photocatalytic Activity of Mn Doped CuFe2O4 Nanoparticles. J Clust Sci 35, 779–798 (2024). https://doi.org/10.1007/s10876-023-02508-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02508-6