Abstract
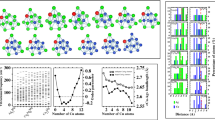
Zn–Cd alloys are important industrial materials which have attracted much attentions in recent years. But, few reports on Zn–Cd clusters are currently available. An interesting structural feature in previous studies is that Zn and Cd atoms do not mix in binary Zn–Cd clusters. However, the analysis based on the empirical potential function can only give very limited structure information about them. Here, geometric structures of (Zn–Cd)n (n = 1–9) clusters are globally searched by the unbiasedly genetic algorithm with DFT methods. We found that the ground state structures of Zn–Cd clusters are singlet states and prefer compact characteristics, where the Cd atoms prefer to be located on the surfaces of the structures, thus coating Zn atoms that are for the kernel growths. The Eb and Δ2E results show that the (Zn–Cd)2 and (Zn–Cd)5 clusters have higher stability than that of their neighbors. The molecular dynamics simulations verify that they still have excellent thermal stability at 700 K. The molecular orbitals and DOS reveal that the 8/20 valence electrons of the (Zn–Cd)2 and (Zn–Cd)5 clusters fill the superatomic shells resulting in electronic configurations of 1S21P6/1S21P61D102S2, respectively. Moreover, we considered different adsorption structures of (Zn–Cd)2 with one CO molecule, and only the four stable isomers are finally obtained. It is found that the CO in the form of molecule is adsorbed on the cluster, resulting in the unbroken C–O bond.





Similar content being viewed by others
Data Availability
The data presented in this study are available upon request from the corresponding authors.
References
O. Schulte and W. B. Holzapfel (1996). Phys. Rev. B 53, 569.
R. Ferrando, J. Jellinek, and R. L. Johnston (2008). Chem. Rev. 108, 845.
R. Bohl and V. Hildebrandt (1957). J. Am. Chem. Soc. 79, 2711.
F. Meydaneri, B. Saatçi, M. Gündüz, and M. Özdemir (2007). Surf. Sci. 601, 2171.
O. Awe and A. Azeez (2017). Appl. Phys. A 123, 1.
R. Hatz, V. Hanninen, and L. Halonen (2014). J. Phys. Chem. A 118, 12274.
L. Amirouche and Ş Erkoç (2004). Phys. Stat. Sol. 241, 292.
L. Amirouche and Ş Erkoç (2005). J. Cryst. Growth 275.
H. Limbu and G. Adhikari (2020). Int. J. Phys. 8, 81.
C. Zanvettor and J. Marques (2014). Chem. Phys. Lett. 608, 373.
Y. Wang, X. Chen, and C. Chen (2021). Inorg. Chem. Commun. 134.
S. J. McCormack and A. Navrotsky (2021). Acta Mater. 202, 1.
O. Akinlade and O. Awe (2006). Int. J. Mater. Res. 97, 377.
A. Pola, M. Tocci, and F. E. Goodwin (2020). Metals 10, 253.
P. Fima and R. Novakovic (2018). Philos. Mag. 98, 1608.
O. Fornaro and H. A. Palacio (2006). Scripta Mater. 54, 2149.
B. Saatçi, M. Ari, M. Gündüz, F. Meydaneri, M. Bozoklu, and S. Durmuş (2006). J. Phys-Condens Mat. 18, 10643.
R. Koirala, B. Singh, I. Jha, and D. Adhikari (2013). J. Mol. Liq. 179, 60.
G. Shrestha and I. Koirala (2021). J. Nepal Phys. Soc. 7, 17.
H. S. Oh, S. J. Kim, K. Odbadrakh, W. H. Ryu, K. N. Yoon, S. Mu, F. Körmann, Y. Ikeda, C. C. Tasan, and D. Raabe (2019). Nat. Commun. 10, 1.
A. Smekhova, A. Kuzmin, K. Siemensmeyer, C. Luo, K. Chen, F. Radu, E. Weschke, U. Reinholz, A. G. Buzanich, and K. V. Yusenko (2022). Nano Res. 15, 4845.
G. L. Hart, T. Mueller, C. Toher, and S. Curtarolo (2021). Nat. Rev. Mater. 6, 730.
S. Sarkar, O. Eriksson, D. Sarma, and I. Di Marco (2022). Phys. Rev. B 105.
A. Lebon, A. Aguado, and A. Vega (2015). Phys. Chem. Chem. Phys. 17, 28033.
R. B. Adamson, C. E. Coleman, and M. Griffiths (2019). J. Nucl. Mater. 521, 167.
V. Roche, G. Koga, T. Matias, C. Kiminami, C. Bolfarini, W. Botta, R. Nogueira, and A. J. Junior (2019). J. Alloys Compd. 774, 168.
C. Yan, K. Sun, J. Huang, S. Johnston, F. Liu, B. P. Veettil, K. Sun, A. Pu, F. Zhou, and J. A. Stride (2017). ACS Energy Lett. 2, 930.
L. Amirouche and Ş Erkoç (2003). Int. J. Mod. Phys. C 14, 905.
L. Amirouche and Ş Erkoç (2003). Phys. Rev. A 68.
A. C. Reber and S. N. Khanna (2017). Acc. Chem. Res. 50, 255.
J. U. Reveles, P. A. Clayborne, A. C. Reber, S. N. Khanna, K. Pradhan, P. Sen, and M. R. Pederson (2009). Nature Chem. 1, 310.
P. Jena and Q. Sun (2018). Chem. Rev. 118, 5755.
D. E. Bergeron, A. W. Castleman Jr., T. Morisato, and S. N. Khanna (2004). Science 304, 84.
S. Heiles, A. J. Logsdail, R. Schäfer, and R. L. Johnston (2012). Nanoscale 4, 1109.
S. G. Neogi and P. Chaudhury (2012). J. Comput. Chem. 33, 629.
S. Ganguly and P. Neogi (2014). J. Comput. Chem. 35, 51.
M. Aslan, J. B. Davis, and R. L. Johnston (2016). Phys. Chem. Chem. Phys. 18, 6676.
Q. Liu, P. Fan, Y. Hu, F. Wang, and L. Cheng (2021). Phys. Chem. Chem. Phys. 23, 10946.
Z. Tian and L. Cheng (2017). J. Phys. Chem. C 121, 20458.
J. Zhao, Q. Du, S. Zhou, and V. Kumar (2020). Chem. Rev. 120, 9021.
J. P. Perdew, K. Burke, and M. Phys (1996). Rev. Lett. 77, 3865.
F. Weigend and R. Ahlrichs (2005). Phys. Chem. Chem. Phys. 7, 3297.
M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, and G. Petersson, Gaussian 09; Revision B. 01 (Gaussian Inc, Wallingford, 2010).
U. Varetto, MOLEKEL version 5.4.0.8 (Swiss National Supercomputing Centre, Manno, Switzerland, 2009)
T. Lu and F. Chen (2012). J. Comput. Chem. 33, 580.
J. Hafner (2008). J. Comput. Chem. 29, 2044.
M. E. Tuckerman, P. J. Ungar, T. Von Rosenvinge, and M. L. Klein (1996). J. Phys. Chem. 100, 12878.
B. Yin and Z. Luo (2021). Coordin Chem. Rev. 429.
H. Häkkinen (2008). Chem. Soc. Rev. 37, 1847.
E. A. Doud, A. Voevodin, T. J. Hochuli, A. M. Champsaur, C. Nuckolls, and X. Roy (2020). Nat. Rev. Mater. 5, 371.
N. V. Tkachenko and A. I. Boldyrev (2019). Phys. Chem. Chem. Phys. 21, 9590.
M. A. Tofanelli, K. Salorinne, T. W. Ni, S. Malola, B. Newell, B. Phillips, H. Häkkinen, and C. J. Ackerson (2016). Chem. Sci. 7, 1882.
Z. Luo, A. Castleman Jr., and S. N. Khanna (2016). Chem. Rev. 116, 14456.
W. T. Wallace and R. L. Whetten (2002). J. Am. Chem. Soc. 124, 7499.
Y. Gao, N. Shao, Y. Pei, Z. Chen, and X. C. Zeng (2011). ACS Nano 5, 7818.
Y. Gao, N. Shao, Y. Pei, and X. C. Zeng (2010). Nano Lett. 10, 1055.
Funding
This work is supported by the Horizontal Cooperation Project of Huainan Normal University (2022HX47) and the Key Project of Scientific Research Foundation of Anhui Province Education Department (KJ2021A0962). The calculations were carried out at the High-Performance Computing Center of Anhui University.
Author information
Authors and Affiliations
Contributions
YM: Methodology, Software, Writing—original draft. QL: Writing - review & editing. LC: Supervision. All authors reviewed the manuscript
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, Y., Liu, Q. & Cheng, L. Structural Features, Superatomic Properties, and Adsorptions of Zn–Cd Nanoalloy. J Clust Sci 35, 159–166 (2024). https://doi.org/10.1007/s10876-023-02472-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02472-1