Abstract
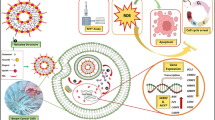
Chemotherapy side effects are minimized, and therapeutic results are enhanced by combination therapy, which is extensively utilized in cancer therapy. MTT experiment showed that compared to the free drugs calreticulin (CRT) and gemcitabine (GEM), GEM-CRT@NPs significantly increased the cytotoxicity of endometrial cancer (Ishikawa cells and KLE cells). These drugs were conjugated to a tri-block copolymeric framework. GEM-CRT@NPs were studied using HR-TEM and DLS analysis to determine particle size and zeta potential. Biochemical staining analyses were also used to investigate the morphological characteristics of endometrial cancer cell lines. After KLE and Ishikawa cancer cells were treated with GEM-CRT@NPs, endometrial cell shape was altered, as evidenced by nuclear fragmentation and nuclear condensation (apoptosis hallmarks). Additionally, the most significant apoptosis ratio to mitochondrial membrane potential indicates that the mitochondrial membrane potential is responsible for the cell's death. Upon treatment with GEM-CRT@NPs, systemic toxicity analysis revealed minimal histological abnormalities and significant blood chemistry alterations, and a near-normal look of the organs. Endometrial cancer treatment may be enhanced with the use of the targeted combination therapeutic system.









Similar content being viewed by others
References
M. Paraskevaidi, C. L. M. Morais, K. M. G. Lima, K. M. Ashton, H. F. Stringfellow, P. L. Martin-Hirsch, and F. L. Martin (2018). Potential of mid-infrared spectroscopy as a non-invasive diagnostic test in urine for endometrial or ovarian cancer. Analyst 143, 3156–3163. https://doi.org/10.1039/C8AN00027A.
L. Feng, J. Li, L. Yang, L. Zhu, X. Huang, S. Zhang, L. Luo, Z. Jiang, T. Jiang, W. Xu, X. Wang, and H. Jin (2017). Tamoxifen activates Nrf2-dependent SQSTM1 transcription to promote endometrial hyperplasia. Theranostics 7, 1890–1900. https://doi.org/10.7150/thno.19135.
Y.-Y. Zhang, F. Zhang, Y.-S. Zhang, K. Thakur, J.-G. Zhang, Y. Liu, H. Kan, and Z.-J. Wei (2019). Mechanism of Juglone-induced cell cycle arrest and apoptosis in Ishikawa human endometrial cancer cells. J. Agric. Food Chem. 67, 7378–7389. https://doi.org/10.1021/acs.jafc.9b02759.
A. Alkhas, B. L. Hood, K. Oliver, P. Teng, J. Oliver, D. Mitchell, C. A. Hamilton, G. L. Maxwell, and T. P. Conrads (2011). Standardization of a sample preparation and analytical workflow for proteomics of archival endometrial cancer tissue. J. Proteome Res. 10, 5264–5271. https://doi.org/10.1021/pr2007736.
L. Philp, H. Chan, M. Rouzbahman, M. Overchuk, J. Chen, G. Zheng, and M. Q. Bernardini (2019). Use of porphysomes to detect primary tumour, lymph node metastases, intra-abdominal metastases and as a tool for image-guided lymphadenectomy: proof of concept in endometrial cancer. Theranostics 9, 2727–2738. https://doi.org/10.7150/thno.31225.
F. Zhang, Y.-Y. Zhang, R.-H. Ma, K. Thakur, J. Han, F. Hu, J.-G. Zhang, and Z.-J. Wei (2021). Multi-omics reveals the anticancer mechanism of asparagus saponin-asparanin A on endometrial cancer Ishikawa cells. Food Funct. 12, 614–632. https://doi.org/10.1039/D0FO02265A.
J. Troisi, L. Sarno, A. Landolfi, G. Scala, P. Martinelli, R. Venturella, A. Di Cello, F. Zullo, and M. Guida (2018). Metabolomic signature of endometrial cancer. J. Proteome Res. 17, 804–812. https://doi.org/10.1021/acs.jproteome.7b00503.
V. Shukla, V. Chandra, P. Sankhwar, P. Popli, J. B. Kaushal, V. K. Sirohi, and A. Dwivedi (2015). Phytoestrogen genistein inhibits EGFR/PI3K/NF-kB activation and induces apoptosis in human endometrial hyperplasial cells. RSC Adv. 5, 56075–56085. https://doi.org/10.1039/C5RA06167A.
X. Shao, G. Ai, L. Wang, J. Qin, Y. Li, H. Jiang, T. Zhang, L. Zhou, Z. Gao, J. Cheng, and Z. Cheng (2019). Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote 27, 367–374. https://doi.org/10.1017/s096719941900042x.
J. Mariscal, P. Fernandez-Puente, V. Calamia, A. Abalo, M. Santacana, X. Matias-Guiu, R. Lopez-Lopez, A. Gil-Moreno, L. Alonso-Alconada, and M. Abal (2019). Proteomic characterization of epithelial-like extracellular vesicles in advanced endometrial cancer. J. Proteome Res. 18, 1043–1053. https://doi.org/10.1021/acs.jproteome.8b00750.
K. Gajjar, J. Trevisan, G. Owens, P. J. Keating, N. J. Wood, H. F. Stringfellow, P. L. Martin-Hirsch, and F. L. Martin (2013). Fourier-transform infrared spectroscopy coupled with a classification machine for the analysis of blood plasma or serum: a novel diagnostic approach for ovarian cancer. Analyst 138, 3917–3926. https://doi.org/10.1039/C3AN36654E.
L. C. Ng and M. Gupta (2020). Transdermal drug delivery systems in diabetes management: a review. Asian J. Pharm. Sci. 15, 13–25. https://doi.org/10.1016/j.ajps.2019.04.006.
O. G. de Jong, S. A. A. Kooijmans, D. E. Murphy, L. Jiang, M. J. W. Evers, J. P. G. Sluijter, P. Vader, and R. M. Schiffelers (2019). Drug delivery with extracellular vesicles: from imagination to innovation. Acc. Chem. Res. 52, 1761–1770. https://doi.org/10.1021/acs.accounts.9b00109.
H. Wu, G. Liu, S. Zhang, J. Shi, L. Zhang, Y. Chen, F. Chen, and H. Chen (2011). Biocompatibility, MR imaging and targeted drug delivery of a rattle-type magnetic mesoporous silica nanosphere system conjugated with PEG and cancer-cell-specific ligands. J. Mater. Chem. 21, 3037–3045. https://doi.org/10.1039/C0JM02863K.
F.-F. An and X.-H. Zhang (2017). Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 7, 3667–3689. https://doi.org/10.7150/thno.19365.
G. I. Frost (2007). Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin. Drug Deliv. 4, 427–440.
K. Zhou, Y. Zhu, X. Chen, L. Li, and W. Xu (2020). Redox- and MMP-2-sensitive drug delivery nanoparticles based on gelatin and albumin for tumor targeted delivery of paclitaxel. Mater. Sci. Eng. C 114, 111006. https://doi.org/10.1016/j.msec.2020.111006.
M. A. Sherwani, S. Tufail, A. A. Khan, and M. Owais (2015). Dendrimer-PLGA based multifunctional immuno-nanocomposite mediated synchronous and tumor selective delivery of siRNA and cisplatin: potential in treatment of hepatocellular carcinoma. RSC Adv. 5, 39512–39531. https://doi.org/10.1039/C5RA03651H.
A. Zhang, K. Jung, A. Li, J. Liu, and C. Boyer (2019). Recent advances in stimuli-responsive polymer systems for remotely controlled drug release. Prog. Polym. Sci. 99, 101164. https://doi.org/10.1016/j.progpolymsci.2019.101164.
Y. Lv, H. He, J. Qi, Y. Lu, W. Zhao, X. Dong, and W. Wu (2018). Visual validation of the measurement of entrapment efficiency of drug nanocarriers. Int. J. Pharm. 547, 395–403. https://doi.org/10.1016/j.ijpharm.2018.06.025.
S. Türk, I. Altınsoy, G. Ç. Efe, M. Ipek, M. Özacar, and C. Bindal (2021). A novel multifunctional NCQDs-based injectable self-crosslinking and in situ forming hydrogel as an innovative stimuli responsive smart drug delivery system for cancer therapy. Mater. Sci. Eng. C 121, 111829. https://doi.org/10.1016/j.msec.2020.111829.
X. Jiang, C. He, and W. Lin (2021). Supramolecular metal-based nanoparticles for drug delivery and cancer therapy. Curr. Opin. Chem. Biol. 61, 143–153. https://doi.org/10.1016/j.cbpa.2021.01.005.
J. Liu, L. Cui, and D. Losic (2013). Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 9, 9243–9257. https://doi.org/10.1016/j.actbio.2013.08.016.
S. T. Dibaba, R. Caputo, W. Xi, J. Z. Zhang, R. Wei, Q. Zhang, J. Zhang, W. Ren, and L. Sun (2019). NIR light-degradable antimony nanoparticle-based drug-delivery nanosystem for synergistic chemo-photothermal therapy in vitro. ACS Appl. Mater. Interfaces 11, 48290–48299. https://doi.org/10.1021/acsami.9b20249.
S. Aryal, H. Park, J. F. Leary, and J. Key (2019). Top-down fabrication-based nano/microparticles for molecular imaging and drug delivery. Int. J. Nanomed. 14, 6631–6644. https://doi.org/10.2147/IJN.S212037.
G. Gallon, V. Lapinte, J.-J. Robin, J. Chopineau, J.-M. Devoisselle, and A. Aubert-Pouëssel (2017). Cross-linked castor oil-based hybrid microparticles as drug delivery systems. ACS Sustain. Chem. Eng. 5, 4311–4319. https://doi.org/10.1021/acssuschemeng.7b00369.
J. Zhou, M. V. Pishko, and J. L. Lutkenhaus (2014). Thermoresponsive layer-by-layer assemblies for nanoparticle-based drug delivery. Langmuir 30, 5903–5910. https://doi.org/10.1021/la501047m.
H. Parhiz, M. Khoshnejad, J. W. Myerson, E. Hood, P. N. Patel, J. S. Brenner, and V. R. Muzykantov (2018). Unintended effects of drug carriers: big issues of small particles. Adv. Drug Deliv. Rev. 130, 90–112. https://doi.org/10.1016/j.addr.2018.06.023.
Y. Tu, F. Peng, A. A. M. André, Y. Men, M. Srinivas, and D. A. Wilson (2017). Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 11, 1957–1963. https://doi.org/10.1021/acsnano.6b08079.
M. M. Wen, N. S. El-Salamouni, W. M. El-Refaie, H. A. Hazzah, M. M. Ali, G. Tosi, R. M. Farid, M. J. Blanco-Prieto, N. Billa, and A. S. Hanafy (2017). Nanotechnology-based drug delivery systems for Alzheimer’s disease management: technical, industrial, and clinical challenges. J. Control Release 245, 95–107.
O. Grinberg, A. Gedanken, C. R. Patra, S. Patra, P. Mukherjee, and D. Mukhopadhyay (2009). Sonochemically prepared BSA microspheres containing gemcitabine, and their potential application in renal cancer therapeutics. Acta Biomater. 5, 3031–3037. https://doi.org/10.1016/j.actbio.2009.05.003.
T. E. Yalcin, S. Ilbasmis-Tamer, and S. Takka (2020). Antitumor activity of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs): in vitro and in vivo. Int. J. Pharm. 580, 119246. https://doi.org/10.1016/j.ijpharm.2020.119246.
L. Yan, G. Liu, H. Cao, H. Zhang, and F. Shao (2019). Hsa_circ_0035483 sponges hsa-miR-335 to promote the gemcitabine-resistance of human renal cancer cells by autophagy regulation. Biochem. Biophys. Res. Commun. 519, 172–178. https://doi.org/10.1016/j.bbrc.2019.08.093.
Z. Jiang, K. Pflug, S. M. Usama, D. Kuai, X. Yan, R. Sitcheran, and K. Burgess (2019). Cyanine-gemcitabine conjugates as targeted theranostic agents for glioblastoma tumor cells. J. Med. Chem. 62, 9236–9245. https://doi.org/10.1021/acs.jmedchem.9b01147.
C.-E. Wu, W.-C. Chou, C.-H. Hsieh, J.W.-C. Chang, C.-Y. Lin, C.-N. Yeh, and J.-S. Chen (2020). Prognostic and predictive factors for Taiwanese patients with advanced biliary tract cancer undergoing frontline chemotherapy with gemcitabine and cisplatin: a real-world experience. BMC Cancer 20, 422. https://doi.org/10.1186/s12885-020-06914-1.
K. Saini, R. S. Prabhuraj, and R. Bandyopadhyaya (2020). Development of mesoporous silica nanoparticles of tunable pore diameter for superior gemcitabine drug delivery in pancreatic cancer cells. J. Nanosci. Nanotechnol. 20, 3084–3096. https://doi.org/10.1166/jnn.2020.17381.
H. Meng, Y. Zhao, J. Dong, M. Xue, Y.-S. Lin, Z. Ji, W. X. Mai, H. Zhang, C. H. Chang, C. J. Brinker, J. I. Zink, and A. E. Nel (2013). Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano 7, 10048–10065. https://doi.org/10.1021/nn404083m.
P. A. Konstantinopoulos, S.-C. Cheng, A. E. Wahner-Hendrickson, R. T. Penson, S. T. Schumer, L. A. Doyle, E. K. Lee, E. C. Kohn, L. R. Duska, M. A. Crispens, A. B. Olawaiye, I. S. Winer, L. M. Barroilhet, S. Fu, M. T. McHale, R. J. Schilder, A. Färkkilä, D. Chowdhury, J. Curtis, R. S. Quinn, B. Bowes, A. D. Dndrea, G. I. Shapiro, and U. A. Matulonis (2020). Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 21, 957–968. https://doi.org/10.1016/S1470-2045(20)30180-7.
X. Li, X. Wang, C. Xu, J. Huang, C. Wang, X. Wang, L. He, and Y. Ling (2015). Synthesis and biological evaluation of nitric oxide-releasing hybrids from gemcitabine and phenylsulfonyl furoxans as anti-tumor agents. MedChemComm 6, 1130–1136. https://doi.org/10.1039/C5MD00158G.
G. K. In and J. Nieva (2015). Emerging chemotherapy agents in lung cancer: nanoparticles therapeutics for non-small cell lung cancer. Transl. Cancer Res. 4 (4), 340.
S. Vallo, R. Köpp, M. Michaelis, F. Rothweiler, G. Bartsch, M. P. Brandt, K. M. Gust, F. Wezel, R. A. Blaheta, A. Haferkamp, and J. Cinatl (2017). Resistance to nanoparticle albumin-bound paclitaxel is mediated by ABCB1 in urothelial cancer cells. Oncol. Lett. 13, 4085–4092. https://doi.org/10.3892/ol.2017.5986.
W. Zhao, J.-S. Wei, P. Zhang, J. Chen, J.-L. Kong, L.-H. Sun, H.-M. Xiong, and H. Möhwald (2017). Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells. ACS Appl. Mater. Interfaces 9, 18474–18481. https://doi.org/10.1021/acsami.7b02542.
Q. Huang, T. W. Johnson, S. Bailey, A. Brooun, K. D. Bunker, B. J. Burke, M. R. Collins, A. S. Cook, J. J. Cui, K. N. Dack, J. G. Deal, Y.-L. Deng, D. Dinh, L. D. Engstrom, M. He, J. Hoffman, R. L. Hoffman, P. S. Johnson, R. S. Kania, H. Lam, J. L. Lam, P. T. Le, Q. Li, L. Lingardo, W. Liu, M. W. Lu, M. McTigue, C. L. Palmer, P. F. Richardson, N. W. Sach, H. Shen, T. Smeal, G. L. Smith, A. E. Stewart, S. Timofeevski, K. Tsaparikos, H. Wang, H. Zhu, J. Zhu, H. Y. Zou, and M. P. Edwards (2014). Design of potent and selective inhibitors to overcome clinical anaplastic lymphoma kinase mutations resistant to crizotinib. J. Med. Chem. 57, 1170–1187. https://doi.org/10.1021/jm401805h.
X. Mao, S. Hu, K. Shang, G. Yang, J. Yan, C. Ma, and J. Yin (2021). Construction of biodegradable core cross-linked nanoparticles from near infrared dyes encoded in polyprodrug amphiphiles and investigation of their synergistic anticancer activity. Polym Chem. https://doi.org/10.1039/D1PY00128K.
G. Picheth, S. Houvenagel, C. Dejean, O. Couture, R. Alves de Freitas, L. Moine, and N. Tsapis (2017). Echogenicity enhancement by end-fluorinated polylactide perfluorohexane nanocapsules: towards ultrasound-activable nanosystems. Acta Biomater. 64, 313–322. https://doi.org/10.1016/j.actbio.2017.10.002.
H. Jin, T. Zhu, X. Huang, M. Sun, H. Li, X. Zhu, M. Liu, Y. Xie, W. Huang, and D. Yan (2019). ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: light-triggered size-reducing and enhanced tumor penetration. Biomaterials 211, 68–80. https://doi.org/10.1016/j.biomaterials.2019.04.029.
M. S. Mohamed Kasim, S. Sundar, and R. Rengan (2018). Synthesis and structure of new binuclear ruthenium(II) arene benzil bis(benzoylhydrazone) complexes: investigation on antiproliferative activity and apoptosis induction. Inorg. Chem. Front. 5, 585–596. https://doi.org/10.1039/c7qi00761b.
T. Sathiya Kamatchi, M. K. Mohamed Subarkhan, R. Ramesh, H. Wang, and J. G. Małecki (2020). Investigation into antiproliferative activity and apoptosis mechanism of new arene Ru(ii) carbazole-based hydrazone complexes. Dalton Trans. 49, 11385–11395. https://doi.org/10.1039/D0DT01476A.
M. K. Mohamed Subarkhan, L. Ren, B. Xie, C. Chen, Y. Wang, and H. Wang (2019). Novel tetranuclear ruthenium(II) arene complexes showing potent cytotoxic and antimetastatic activity as well as low toxicity in vivo. Eur. J. Med. Chem. 179, 246–256. https://doi.org/10.1016/j.ejmech.2019.06.061.
M. K. M. Subarkhan and R. Ramesh (2016). Ruthenium(II) arene complexes containing benzhydrazone ligands: synthesis, structure and antiproliferative activity. Inorg. Chem. Front. 3, 1245–1255. https://doi.org/10.1039/c6qi00197a.
S. Balaji, M. K. Mohamed Subarkhan, R. Ramesh, H. Wang, and D. Semeril (2020). Synthesis and structure of arene Ru(II) NO-chelating complexes: in vitro cytotoxicity and cancer cell death mechanism. Organometallics 39, 1366–1375. https://doi.org/10.1021/acs.organomet.0c00092.
N. Mohan, M. K. Mohamed Subarkhan, and R. Ramesh (2018). Synthesis, antiproliferative activity and apoptosis-promoting effects of arene ruthenium(II) complexes with N, O chelating ligands. J Organomet Chem. https://doi.org/10.1016/j.jorganchem.2018.01.022.
M. K. Mohamed Subarkhan, R. Ramesh, and Y. Liu (2016). Synthesis and molecular structure of arene ruthenium(II) benzhydrazone complexes: Impact of substitution at the chelating ligand and arene moiety on antiproliferative activity. New J Chem. https://doi.org/10.1039/c6nj01936f.
X. Li and Y. Gao (2020). Synergistically fabricated polymeric nanoparticles featuring dual drug delivery system to enhance the nursing care of cervical cancer. Process Biochem. 98, 254–261. https://doi.org/10.1016/j.procbio.2020.09.010.
K. Liu, P. Liu, R. Liu, and X. Wu (2015). Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 21, 15–20. https://doi.org/10.12659/MSMBR.893327.
S. Kasibhatla, G. P. Amarante-Mendes, D. Finucane, T. Brunner, E. Bossy-Wetzel, and D. R. Green (2006). Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. 2006, 799–803. https://doi.org/10.1101/pdb.prot4493.
D. Fischer, Y. Li, B. Ahlemeyer, J. Krieglstein, and T. Kissel (2003). In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 24, 1121–1131. https://doi.org/10.1016/S0142-9612(02)00445-3.
W. Wang, X. Dong, Y. Liu, B. Ni, N. Sai, L. You, M. Sun, Y. Yao, C. Qu, X. Yin, and J. Ni (2020). Itraconazole exerts anti-liver cancer potential through the Wnt, PI3K/AKT/mTOR, and ROS pathways. Biomed. Pharmacother. 131, 110661. https://doi.org/10.1016/j.biopha.2020.110661.
A. Nagarsenkar, L. Guntuku, S. D. Guggilapu, B. K. Danthi, S. Gannoju, V. G. M. Naidu, and N. B. Bathini (2016). Synthesis and apoptosis inducing studies of triazole linked 3-benzylidene isatin derivatives. Eur. J. Med. Chem. 124, 782–793. https://doi.org/10.1016/j.ejmech.2016.09.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Tt., Yuan, Mm., Tao, Ym. et al. Engineering of Self-assembly Polymers Encapsulated with Dual Anticancer Drugs for the Treatment of Endometrial Cancer. J Clust Sci 33, 2661–2671 (2022). https://doi.org/10.1007/s10876-021-02175-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02175-5