Abstract
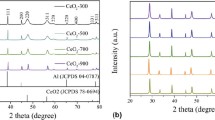
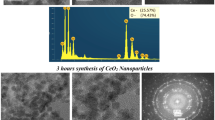
Metal oxide nanoparticles have been used for numerous applications in industry. The cerium oxide (CeO2) NPs were synthesized in the supercritical water (SCW) using its nitrate salt as a precursor. The response surface methodology (RSM) with the central composite design (CCD) was used to model the data as well as to optimize the synthesis procedure. The effect of different process parameters including reaction temperature (400–500 °C), concentration of the precursor solution (0.1–0.5 M), and reaction time (20–100 min) on the crystallite size and yield of the synthesis was studied. The results suggest that the precursor solution concentration had a significant effect on both the crystallite size and the reaction yield, while the temperature and reaction time significantly affected the crystallite size and the reaction yield, respectively. The achieved optimal conditions for the synthesis of CeO2 NPs were 500 °C, a reaction time of 73.26 min, and an initial precursor concentration of 0.28 M, where a crystallite size of 21.19 nm and a yield of 87.53% were achieved.


Similar content being viewed by others
References
Z. Bo, S. Ahn, M.A. Ardagh, N.M. Schweitzer, C.P. Canlas, O.K. Farha, J.M. Notestein, Synthesis and stabilization of small Pt nanoparticles on TiO2 partially masked by SiO2, Applied Catalysis A: General, 551 (2018) 122–128.
Z. Xiao, Design and preparation of hydrated MgAl supported Cu catalysts with high alkalinity by MOCVD for the hydrogenolysis of cellulose, Applied Catalysis A: General, 542 (2017) 343–349.
C. Wang, T. Qu, D. Wang, Z. Kang, Synthesis of Co-Fe-Pd nanoparticles via ultrasonic irradiation and their electro-catalytic activity for oxygen reduction reaction, Applied Catalysis A: General, 560 (2018) 103–110.
M. Kosari, M. Golmohammadi, S.J. Ahmadi, J. Towfighi, A.H. Chenari, On the catalysis capability of transition metal oxide nanoparticles in upgrading of heavy petroleum residue by supercritical water, The Journal of Supercritical Fluids, 126 (2017) 14–24.
T. Adschiri, Y.-W. Lee, M. Goto, S. Takami, Green materials synthesis with supercritical water, Green Chemistry, 13 (2011) 1380–1390.
T. Adschiri, Y. Hakuta, K. Sue, K. Arai, Hydrothermal synthesis of metal oxide nanoparticles at supercritical conditions, Journal of Nanoparticle Research, 3 (2001) 227–235.
H. Hayashi, Y. Hakuta, Hydrothermal synthesis of metal oxide nanoparticles in supercritical water, Materials, 3 (2010) 3794–3817.
N. Akiya, P.E. Savage, Roles of water for chemical reactions in high-temperature water, Chemical reviews, 102 (2002) 2725–2750.
P.E. Savage, Organic chemical reactions in supercritical water, Chemical reviews, 99 (1999) 603–622.
T. Adschiri, K. Kanazawa, K. Arai, Rapid and continuous hydrothermal crystallization of metal oxide particles in supercritical water, Journal of the American Ceramic Society, 75 (1992) 1019–1022.
S. Takami, T. Sato, T. Mousavand, S. Ohara, M. Umetsu, T. Adschiri, Hydrothermal synthesis of surface-modified iron oxide nanoparticles, Materials Letters, 61 (2007) 4769–4772.
M. Golmohammadi, S.J. Ahmadi, J. Towfighi, Catalytic cracking of heavy petroleum residue in supercritical water: Study on the effect of different metal oxide nanoparticles, The Journal of Supercritical Fluids, 113 (2016) 136–143.
M. Kosari, M. Golmohammadi, J. Towfighi, S.J. Ahmadi, Decomposition of tributhyl phosphate at supercritical water oxidation conditions: Non-catalytic, catalytic, and kinetic reaction studies, The Journal of Supercritical Fluids, 133 (2018) 103–113.
G. Du, Y. Yang, W. Qiu, S. Lim, L. Pfefferle, G.L. Haller, Statistical design and modeling of the process of methane partial oxidation using V-MCM-41 catalysts and the prediction of the formaldehyde production, Applied Catalysis A: General, 313 (2006) 1–13.
S.J. Nejad, A. Golzary, Experimental design for the optimization of hydrothermal synthesis of samarium oxide (Sm2O3) nanoparticles under supercritical water condition, International Journal of Chemical Engineering and Applications, 2 (2011) 243.
M. Outokesh, M. Hosseinpour, S. Ahmadi, T. Mousavand, S. Sadjadi, W. Soltanian, Hydrothermal synthesis of CuO nanoparticles: study on effects of operational conditions on yield, purity, and size of the nanoparticles, Industrial & Engineering Chemistry Research, 50 (2011) 3540–3554.
S.J. Ahmadi, M. Hosseinpour, F. Javadi, R. Tayebee, Optimization study on formation and decomposition of zinc hydroxynitrates to pure zinc oxide nanoparticles in supercritical water, Industrial & Engineering Chemistry Research, 52 (2013) 1448–1454.
O. Karhan, O.z.B. Ceran, O.N. Sara, B. Şimşek, Response Surface Methodology Based Desirability Function Approach To Investigate Optimal Mixture Ratio of Silver Nanoparticles Synthesis Process, Industrial & Engineering Chemistry Research, 56 (2017) 8180–8189.
S. Masoumi, J. Towfighi, A. Mohamadalizadeh, Z. Kooshki, K. Rahimi, Tri-templates synthesis of SAPO-34 and its performance in MTO reaction by statistical design of experiments, Applied Catalysis A: General, 493 (2015) 103–111.
S. Petrovic, L. Rozic, V. Jovic, S. Stojadinovic, B. Grbic, N. Radic, J. Lamovec, R. Vasilic, Optimization of a nanoparticle ball milling process parameters using the response surface method, Advanced Powder Technology, (2018).
V. Srivastava, Y. Sharma, M. Sillanpää, Application of response surface methodology for optimization of Co (II) removal from synthetic wastewater by adsorption on NiO nanoparticles, Journal of Molecular Liquids, 211 (2015) 613–620.
M. Golmohammadi, J. Towfighi, M. Hosseinpour, S.J. Ahmadi, An investigation into the formation and conversion of metal complexes to metal oxide nanoparticles in supercritical water, The Journal of Supercritical Fluids, 107 (2016) 699–706.
M. Golmohammadi, S.J. Ahmadi, J. Towfighi, Catalytic supercritical water destructive oxidation of tributyl phosphate: Study on the effect of operational parameters, The Journal of Supercritical Fluids, (2018).
H. Li, C. Li, C. Jiao, S. Wang, Characterization of cerium dioxide nanoparticles prepared by supercritical water oxidation, Ceramics International, 41 (2015) 10170–10176.
H. Wang, J.-R. Zhang, J.-J. Zhu, A microwave assisted heating method for the rapid synthesis of sphalrite-type mercury sulfide nanocrystals with different sizes, Journal of Crystal Growth, 233 (2001) 829–836.
R.O. Fuentes, J.D. Woollins, R.T. Baker, Temperature effects on structural properties in the synthesis of nanocrystalline Zr0. 5Ce0. 5O2 solid solution: A study by XRD and HRTEM, Journal of Alloys and Compounds, 495 (2010) 565–569.
B. Stuart, Infrared spectroscopy, Kirk‐Othmer Encyclopedia of Chemical Technology, (2005).
B. Yan, H. Zhu, Controlled synthesis of CeO 2 nanoparticles using novel amphiphilic cerium complex precursors, Journal of Nanoparticle Research, 10 (2008) 1279–1285.
J.-R. Kim, W.-J. Myeong, S.-K. Ihm, Characteristics of CeO 2–ZrO 2 mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as support of Rh catalyst for catalytic reduction of NO by CO, Journal of Catalysis, 263 (2009) 123–133.
K.S. Sing, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984), Pure and applied chemistry, 57 (1985) 603–619.
W.-S. Son, T.J. Yoon, H.J. Park, M. Kim, T. Adschiri, Y.-W. Lee, A novel sample preparation method on CeO2 nanoparticles with TEM grid embedded liquid CO2 displacement and supercritical CO2 drying for microscopic analysis, The Journal of Supercritical Fluids, 152 (2019) 104559.
C.d. Slostowski, S. Marre, O. Babot, T. Toupance, C. Aymonier, Near-and supercritical alcohols as solvents and surface modifiers for the continuous synthesis of cerium oxide nanoparticles, Langmuir, 28 (2012) 16656–16663.
Acknowledgement
The authors are grateful for the financial support from the Birjand University of Technology and the technical assistance from the technicians in our department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Golmohammadi, M., Rahmati-Abkenar, M. & Ghanbari, S. A Facile Method for the Synthesis of Metal Oxide Nanoparticles in Supercritical Water: Optimized Procedure for Cerium Oxide. J Clust Sci 33, 887–893 (2022). https://doi.org/10.1007/s10876-021-02007-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02007-6