Abstract
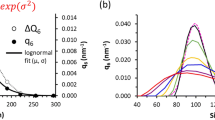
Particle size distribution of nanoparticles plays an important role in modelling many scientific and engineering problems. In this article, we proposed a Finite Volume Method (FVM) to model TiO2 nanoparticles formation using population balance equations (PBEs) by incorporating the simultaneous agglomeration and disintegration processes. The superposition of the PBEs for agglomeration and disintegration with different kernels leads to a system of partial-integro differential equations, which are numerically solved by using FVM. The precipitation of TiO2 nanoparticles in the batch reactor is studied experimentally as well as by numerical simulations based on Austin and Diemer disintegration kernels and Shear agglomeration kernel. Finally, the capability of the precipitation model is evaluated and the experimental results on particle sizes are compared with the numerical results.




Similar content being viewed by others
Abbreviations
- B:
-
Disintegration function (m−3)
- D:
-
Particle size (µ)
- S:
-
Selection function (s−1)
- T:
-
Time (s)
- x, y:
-
Particle volume (m3)
- Qr (d):
-
Cumulative particle size distribution (%)
- Re:
-
Reynolds-Number
- β:
-
One-dimensional agglomeration kernel (m−3. s−1)
- ε:
-
Turbulent energy dissipation rate (m2. s−3)
- \( \upsilon \) :
-
Kinematic viscosity of the fluid (m2. s−1)
- \( \eta \) :
-
Viscosity of the fluid (kg. m−1. s−1)
- λ:
-
Wavelength (m)
- \( \dot{\gamma } \) :
-
Shear rate (s−1)
- \( \,\phi \) :
-
Dimensionless material constant
- \( \gamma \) :
-
Dimensionless material constant
References
A. Hagfeldt and M. Graetzel (2002). Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 95, (1), 49–68.
S. Sarangapani, B. V. Tilak, and C. P. Chen (1996). Materials for electrochemical capacitors theoretical and experimental constraints. J. Electrochem. Soc. 143, (11), 3791–3799.
L. Vayssières, C. Chanéac, E. Tronc, and J. P. Jolivet (1998). Size tailoring of magnetite particles formed by aqueous precipitation: an example of thermodynamic stability of nanometric oxide particles. J. Colloid Interface Sci. 205, (2), 205–212.
H. C. Schwarzer and W. Peukert (2005). Prediction of aggregation kinetics based on surface properties of nanoparticles. Chem. Eng. Sci. 60, (1), 11–25.
H. C. Schwarzer and W. Peukert (2002). Experimental investigation into the influence of mixing on nanoparticle precipitation. Chem. Eng. Technol. 25, (6), 657–661.
J. T. T. Nikolov, W. Hintz, and V. Jordanova (2003). Synthesis and characterization of titanium dioxide nanoparticles. J. Univ. Chem. Technol. Metall. 3, 725–734.
B. J. Ridder, A. Majumder, and Z. K. Nagy (2014). Population balance model-based multiobjective optimization of a multisegment multiaddition (MSMA) continuous plug-flow antisolvent crystallizer. Ind. Eng. Chem. Res. 53, (11), 4387–4397.
R. T. Kügler, S. Doyle, and M. Kind (2015). Fundamental insights into barium sulfate precipitation by time-resolved in situ synchrotron radiation wide-angle X-ray scattering (WAXS). Chem. Eng. Sci. 133, 140.
H. C. Schwarzer, F. Schwertfirm, M. Manhart, H. J. Schmid, and W. Peukert (2006). Predictive simulation of nanoparticle precipitation based on the population balance equation. Chem. Eng. Sci. 61, (1), 167–181.
M. C. Heine and S. E. Pratsinis, “Droplet and particle dynamics during flame spray synthesis of nanoparticles,” in AIChE Annual Meeting, Conference Proceedings, 2005, p. 3046.
S. R. K. Perala and S. Kumar (2014). On the two-step mechanism for synthesis of transition-metal nanoparticles. Langmuir 30, (42), 12703–12711.
P. Stolzenburg and G. Garnweitner (2017). Experimental and numerical insights into the formation of zirconia nanoparticles: a population balance model for the nonaqueous synthesis. React. Chem. Eng. 2, (3), 337–348.
D. Ramkrishna Population balances: theory and applications to particulate systems in engineering (Academic Press, Cambridge, 2000).
Y. P. Gokhale, R. Kumar, J. Kumar, W. Hintz, G. Warnecke, and J. Tomas (2009). Disintegration process of surface stabilized sol-gel TiO 2 nanoparticles by population balances. Chem. Eng. Sci. 64, (24), 5302–5307.
R. Kumar, J. Kumar, and G. Warnecke (2012). Moment presering finite volume schemes for solving population balance equations incorporating aggregation, breakage, growth and source terms. Math. Model. Methods Appl. Sci. 23, (07), 1235–1273.
Z. A. Melzak (1957). A scalar transport equation. Trans. Am. Math. Soc. 85, (2), 547.
R. M. Ziff (1991). New solutions to the fragmentation equation. J. Phys. A Gen. Phys. 24, (12), 2821–2828.
D. L. Marchisio and R. O. Fox (2005). Solution of population balance equations using the direct quadrature method of moments. J. Aerosol Sci. 36, (1), 43–73.
F. Filbet and P. Laurençot (2004). Numerical simulation of the Smoluchowski coagulation equation. SIAM J. Sci. Comput. 25, (6), 2004–2028.
J. Kumar, M. Peglow, G. Warnecke, and S. Heinrich (2008). An efficient numerical technique for solving population balance equation involving aggregation, breakage, growth and nucleation. Powder Technol. 182, (1), 81–104.
S. Kumar and D. Ramkrishna (1996). On the solution of population balance equations by discretization—I. A fixed pivot technique. Chem. Eng. Sci. 51, (8), 1311–1332.
M. Vanni (2000). Approximate population balance equations for aggregation-breakage processes. J. Colloid Interface Sci. 221, (2), 143–160.
M. Kostoglou and A. J. Karabelas (2009). On sectional techniques for the solution of the breakage equation. Comput. Chem. Eng. 33, (1), 112–121.
W. Hundsdorfer and J. G. Verwer Numerical solution of time-dependent advection-diffusion-reaction equations, 1st ed (Springer-Verlag, Berlin Heidelberg, 2003).
R. Kumar and J. Kumar (2013). Numerical simulation and convergence analysis of a finite volume scheme for solving general breakage population balance equations. Appl. Math. Comput. 219, (10), 5140–5151.
L. G. Austin (2002). A treatment of impact breakage of particles. Powder Technol. 126, (1), 85–90.
R. B. Diemer and J. H. Olson (2002). A moment methodology for coagulation and breakage problems: part 1—analytical solution of the steady-state population balance. Chem. Eng. Sci. 57, (12), 2193–2209.
R. B. Diemer and J. H. Olson (2002). A moment methodology for coagulation and breakage problems: part 3-generalized daughter distribution functions. Chem. Eng. Sci. 57, (19), 4187–4198.
P. G. Saffman and J. S. Turner (1956). On the collision of drops in turbulent clouds. J. Fluid Mech. 1, (1), 16–30.
M. Sommer, F. Stenger, W. Peukert, and N. J. Wagner (2006). Agglomeration and breakage of nanoparticles in stirred media mills—a comparison of different methods and models. Chem. Eng. Sci. 61, (1), 135–148.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, R., Gokhale, Y.P. & Surasani, V.K. Population Balance Modeling with Coupled Agglomeration and Disintegration Processes for TiO2 Nanoparticles Formation and Experimental Validation. J Clust Sci 32, 1361–1369 (2021). https://doi.org/10.1007/s10876-020-01895-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01895-4