Abstract
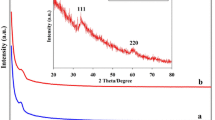
Facile and clean transformation for synthesizing secondary arylamines through one-pot reductive amination of aniline has been catalyzed by supported nickel nanocluster and poly(vinylsulfonic acid) into mesoporous carbon CMK-3 (Ni/PVSA/CMK-3) as a bi-functional metal/acid heterogeneous catalyst. Vinylsulfonic acid has been polymerized in CMK-3 pores by an in situ method. The process was run in the presence of NaBH4 at room temperature, in a short reaction time, and without secondary product. Various characterization techniques, including FT-IR, XRD, TG, BET, SEM, TEM, DRS-UV, and AAS were employed to disclose the physical and chemical properties of the catalyst. Reaction results demonstrate that the optimized Ni/PVSA/CMK-3 catalyst shows comparable catalytic performance thanks to the nickel metals and the acidic nature of polymer incorporated in mesoporous channels of CMK-3. Besides being eco-friendly (using water as solvent), the method has several advantages such as simple work-up procedure and moderate to high-yield. This catalyst was easily filtered and reused without noticeable loss of activity after 10 runs.















Similar content being viewed by others
References
S. Gomez, J. A. Peters, and T. Maschmeyer (2002). Adv. Synth. Catal. 344, 1037.
E. W. Baxter and A. B. Reitz (2004). Org. React. 59, 1.
A. F. Abdel-Magid and S. J. Mehrman (2006). Org. Process Res. Dev. 10, 971.
Y. Z. Chen, Y. X. Zhou, H. Wang, J. Lu, T. Uchida, Q. Xu, S. H. Yu, and H. L. Jiang (2015). ACS Catal. 5, 2062.
M. Nasrollahzadeh and S. M. Sajadi (2016). J. Colloid Interface Sci. 464, 147.
J. W. Park and Y. K. Chung (2015). ACS Catal. 5, 4846.
C. C. Lee and S. T. Liu (2011). Chem. Commun. 47, 6981.
M. H. Valkenberg and W. F. Holderich (2002). Catal. Rev. 44, 321.
Q. Zhang, S. S. Li, M. M. Zhu, Y. M. Liu, H. Y. He, and Y. Cao (2016). Green Chem. 18, 2507.
A. Saha and B. Ranu (2008). J. Org. Chem. 73, 6867.
A. Rahman and S. B. Jonnalagadda (2008). Catal. Lett. 123, 264.
R. Rajesh and R. Venkatesan (2012). J. Mol. Catal. A Chem. 359, 88.
F. Alonso, P. Riente, and M. Yus (2008). Synlett 9, 1289.
F. Alonso, P. Riente, and M. Yus (1998). Tetrahedron 54, 1921.
D. Shah and H. Kaur (2014). J. Mol. Catal. A Chem. 381, 70.
L. F. Giraldo, B. L. López, L. Pérez, S. Urrego, L. Sierra, and M. Mesa (2007). Macromol. Symp. 258, 129.
O. Mazaheri and R. J. Kalbasi (2015). RSC Adv. 5, 34398.
S. K. Jain, R. J. M. Pellenq, K. E. Gubbins, and X. Peng (2017). Langmuir 33, 2109.
A. Eftekhari and Z. Fan (2017). Mater. Chem. Front. 1, 1001.
M. Choi and R. Ryoo (2003). Nat. Mater. 2, 473.
S. Jun, S. H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna, and O. Terasaki (2000). J. Am. Chem. Soc. 122, 10712.
M. Colilla, F. Balas, M. Manzano, and M. Vallet-Regí (2007). Chem. Mater. 19, 3099.
R. J. Kalbasi and O. Mazaheri (2016). New J. Chem. 40, 9627.
J. He, K. Ma, J. Jin, Z. Dong, J. Wang, and R. Li (2009). Microporous Mesoporous Mater. 121, 173.
T. Okayasu, K. Saito, H. Nishide, and M. T. W. Hearn (2009). Chem. Commun. 0, 4708.
M. Erkartal, H. Usta, M. Citir, and U. Sen (2016). J. Membr. Sci. 499, 156.
D. Li, J. Li, D. Mao, H. Wen, Y. Zhou, and J. Wang (2017). Mater. Chem. Phys. 189, 118.
D. D. Jiang, Q. Yao, M. A. McKinney, and C. A. Wilkie (1999). Polym. Degrad. Stab. 63, 423.
K. H. Wu, Y. R. Wang, and W. H. Hwu (2003). Polym. Degrad. Stab. 79, 195.
R. J. Kalbasi and N. Mosaddegh (2011). J. Solid State Chem. 184, 3095.
S. Chytil, W. R. Glomm, E. Vollebekk, H. Bergem, J. Walmsley, J. Sjoblom, and E. A. Blekkan (2005). Microporous Mesoporous Mater. 86, 198.
H. Song, R. Rioux, J. D. Hoefelmeyer, R. Komor, K. Niesz, M. Grass, P. Yang, and G. A. Somorjai (2006). J. Am. Chem. Soc. 128, 3027.
P. Dibandjo, F. Chassagneux, L. Bois, C. Sigala, and P. Miele (2005). J. Mater. Chem. 15, 1917.
D. Brühwiler and H. Frei (2003). J. Phys. Chem. B. 107, 8547.
S. Kim, B. K. Yoo, K. Chun, W. Kang, J. Choo, M. S. Gong, and S. W. Joo (2005). J. Mol. Catal. A Chem. 226, 231.
R. Jana, T. P. Pathak, and M. S. Sigman (2011). Chem. Rev. 111, 1417.
E. Levin, E. Ivry, C. E. Diesendruck, and N. G. Lemcoff (2015). Chem. Rev. 115, 4607.
H. Wena, K. Yao, Y. Zhang, Z. Zhou, and A. Kirschning (2009). Catal. Commun. 19, 1207.
O. Metin and S. Ozkar (2008). J. Mol. Catal. A Chem. 295, 39.
R. J. Kalbasi and F. Zamani (2014). RSC Adv. 4, 7444.
R. J. Kalbasi, N. Mosaddegh, and A. Abbaspourrad (2012). Appl. Catal. A Gen. 78, 423–424.
R. J. Kalbasi and O. Mazaheri (2015). Catal. Commun. 69, 86.
M. A. Harrad, B. Boualy, L. El Firdoussi, A. Mehdi, C. Santi, S. Giovagnoli, M. Nocchetti, and M. Ait Ali (2013). Catal. Commun. 32, 92.
Y. Zhai, Y. Dou, X. Liu, S. S. Park, C. S. Ha, and D. Zhao (2011). Carbon 49, 545.
S. E. Bozbag, L. C. Zhang, M. Aindow, and C. Erkey (2012). J. Supercrit. Fluids 66, 265.
N. Uday Kumar, B. Sudhakar Reddy, V. Prabhakar Reddy, and R. Bandichhor (2012). Tetrahedron Lett. 53, 4354.
R. P. Tripathi, S. S. Verma, J. Pandey, and V. K. Tiwari (2008). Curr. Org. Chem. 12, 1093.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Javad Kalbasi, R., Parishani, P. & Mazaheri, O. Encapsulation of Nickel Nanoparticles and Homopoly(Vinylsulfonic Acid) in Mesoporous Carbon CMK-3 as an Acid–Metal Bifunctional Catalyst for Tandem Reductive Amination. J Clust Sci 29, 561–575 (2018). https://doi.org/10.1007/s10876-018-1366-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1366-6