Abstract
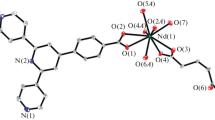
The title complex La/3-methylbenzoic acid (HMeBA) with o-phenanthroline has been hydrothermally synthesized directly: [La3(MeBA)8(OH)(o-Phen)(H2O)]n 1 (o-Phen = o-phenanthroline). The crystal structure was determined by single-crystal X-ray diffraction with the following data: triclinic P − 1. The complex is a one-dimensional chain and one water molecule and [OH]− also coordinate with La ion in particular. Interestingly, the one-dimensional chain is composed of the novel repeated La6 cluster units. Additionally, in order to explore the structural characteristic, 2D correlation analysis of FTIR with thermal perturbation, photo-luminescent and solid UV–Vis spectrum are used.









Similar content being viewed by others
References
C. C. Shan, M. Y. Rong, G. Z. Zhen, and M. C. Shu (2010). Cryst. Growth Des. 10, 1155.
V. E. Emily, R. H. Sanchez, and T. A. Betley (2013). Inorg. Chem. 52, 5006.
T. Julia, K. Carlos, and D. Sixto (2008). Pure Appl. Chem. 80, 1303.
C. H. Zhang, Y. G. Chen, Q. Tang, and S. X. Liu (2012). Dalton Trans. 41, 9971.
A. F. Williams (2009). Chem. Met. Alloys. 2, 1.
R. M. Hernandez and N. Molina (2012). Russ. J. Coord. Chem. 38, 159.
L. Zhang, C. Wang, and X. Wu (2011). Russ. J. Coord. Chem. 37, 382.
M. Andruh (2007). Chem. Commun. 25, 2565.
E. J. L. Mclnnes, S. Piligkos, G. A. Timco, and R. E. P. Winpenny (2005). Coord. Chem. Rev. 249, 2577.
M. W. Cooke and G. S. Hanan (2007). Chem. Soc. Rev. 36, 1466.
L. M. C. Beltran and J. R. Long (2005). Acc. Chem. Res. 38, 325.
M. Manoli, A. Prescimone, and R. Bagai (2007). Inorg. Chem. 460, 6968.
A. Dolbecq and F. Secheresse (2002). Adv. Inorg. Chem. 53, 1.
J. L. Atwood, L. J. Barbour, M. J. Hardie, and C. L. Raston (2001). Coord. Chem. Rev. 222, 3.
S. Aime, M. Botta, M. Fasano, and E. Terreno (1999). Chem. Soc. Rev. 27, 19.
M. Komiyama, N. Takeda, and H. Shigekawa (1999). Chem. Commun. 1443.
P. C. Andrews, T. Beck, and C. M. Forsyth (2007). Dalton Trans. 48, 5651.
M. Romanelli, G. A. Kumar, and T. Emge (2008). J. Angew. Chem. Int. Ed. 47, 6049.
K. L. Wong (2004). Angew. Chem. Int. Ed. 43, 4659.
M. D. Vaira (2003). Inorg. Chem. 42, 3157.
J. Y. Niu (2004). Eur. J. Inorg. Chem. 160.
(a) Sheldrick, G.M(1996). SADABS, University of Gottingen: Gottingen, Germany. (b) Sheldrick, G.M (1997). SHELXS 97, Program for the Solution of Crystal Structures; University of Gottingen: Gottingen, Germany.
R. Baggio, M. T. Garland, and O. Pen (2005). Inorg. Chim. Acta. 358, 2332.
L. Y. Zhao, X. C. Chai, and Y. P. Chen (2014). Synth. React. Inorg. Met. Org. Nano Met. Chem. 44, 572.
H. K.C, G. L. S (1999). Bull. Korean Chem. Soc. 20, 417.
X. F. Li, R. Cao, and Y. F. Li (2009). Inorg. Chem. Commun. 12, 667.
Z. Yang, Y. X. Wang, A. M. Guo, H. J. Zhu, and S. Xiong (2009). J. Chem. Phys. 359, 82.
P. Koczon, J. M. Piekut, and W. Borawska (2003). J. Mol. Struct. 651, 651.
K. Nakamoto (Wiley Interscience, New York, 1986).
X. C. Chai, H. H. Zhang, and S. Zhang (2009). J. Solid State Chem. 182, 1889.
Y. L. Zhao and F. Y. Zhao (2002). Spectrosc. Spect. Anal. 22, 987.
Y. P. Chen, X. M. Shen, and H. H. Zhang (2006). Vib. Spectrosc. 40, 142.
I. Noda and Y. Ozaki (John Wiley & Sons, Ltd, 2004.
L. J. Bellamy (Wiley, New York, 1958).
S. Zhang, Y. N. Cao, and H. H. Zhang (2008). J. Solid State Chem. 181, 399.
R. Lei, X. C. Chai, and H. X. Mei (2010). J. Solid State Chem. 183, 1510.
I. Noda (1993). Appl. spectrosc. 47, 1329.
Y. Q. Xu, D. Q. Yuan, and B. L. Wu (2005). Inorg. Chem. Commun. 8, 651.
G. P. Yong, Z. Y. Wang, and Y. Cui (2004). Eur. J. Inorg. Chem. 21, 431.
L. Y. Zhao, X. C. Chai, and Y. P. Chen (2014). Synth. React. Inorg. M. 44, 572.
X. X. Chen, Y. N. Cao, and H. H. Zhang (2008). J. Solid State Chem. 181, 1133.
N. S. Deb, D. Baruah, N. S. Sarma, and N. S. Dass (1998). Thermochim. Acta. 320, 53.
Acknowledgements
The project was supported by Scientific Research Fund of Hebei Provincial Education Department (z2017017). We thank Prof. Sun Suqin (Tsinghua University, Beijing, China) for providing the 2D IR correlation analysis software.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, LY., Yang, YX., Deng, XC. et al. Synthesis, Characterization and Crystal Structure of Polymer [La3(MeBA)8(OH)(o-Phen)(H2O)]n Containing the Novel La6 Cluster Unit. J Clust Sci 28, 3115–3126 (2017). https://doi.org/10.1007/s10876-017-1277-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1277-y