Abstract
Background
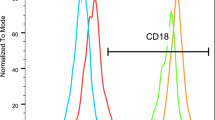
Leukocyte and platelet integrin function defects are present in leukocyte adhesion deficiency type III (LAD-III) due to mutations in FERMT3. Additionally, osteoclast/osteoblast dysfunction develops in LAD-III.
Aim
To discuss the distinguishing clinical, radiological, and laboratory features of LAD-III.
Methods
This study included the clinical, radiological, and laboratory characteristics of twelve LAD-III patients.
Results
The male/female ratio was 8/4. The parental consanguinity ratio was 100%. Half of the patients had a family history of patients with similar findings. The median age at presentation and diagnosis was 18 (1–60) days and 6 (1–20) months, respectively. The median leukocyte count on admission was 43,150 (30,900–75,700)/μL. The absolute eosinophil count was tested in 8/12 patients, and eosinophilia was found in 6/8 (75%). All patients had a history of sepsis. Other severe infections were pneumonia (66.6%), omphalitis (25%), osteomyelitis (16.6%), gingivitis/periodontitis (16%), chorioretinitis (8.3%), otitis media (8.3%), diarrhea (8.3%), and palpebral conjunctiva infection (8.3%). Four patients (33.3%) received hematopoietic stem cell transplantation (HSCT) from HLA-matched-related donors, and one deceased after HSCT. At initial presentation, 4 (33.3%) patients were diagnosed with other hematologic disorders, three patients (P5, P7, and P8) with juvenile myelomonocytic leukemia (JMML), and one (P2) with myelodysplastic syndrome (MDS).
Conclusion
In LAD-III, leukocytosis, eosinophilia, and bone marrow findings may mimic pathologies such as JMML and MDS. In addition to non-purulent infection susceptibility, patients with LAD-III exhibit Glanzmann-type bleeding disorder. In LAD-III, absent integrin activation due to kindlin-3 deficiency disrupts osteoclast actin cytoskeleton organization. This results in defective bone resorption and osteopetrosis-like radiological changes. These are distinctive features compared to other LAD types.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Yaz I, Ozbek B, Bildik HN, Tan C, Oskay Halacli S, Soyak Aytekin E, et al. Clinical and laboratory findings in patients with leukocyte adhesion deficiency type I: a multicenter study in Turkey. Clin Exp Immunol. 2021;206(1):47–55.
Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–14.
Kuijpers TW, van Bruggen R, Kamerbeek N, Tool AT, Hicsonmez G, Gurgey A, et al. Natural history and early diagnosis of LAD-1/variant syndrome. Blood. 2007;109(8):3529–37.
Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. 2012;1250(1):50–5.
Cagdas D, Yılmaz M, Kandemir N, Tezcan İ, Etzioni A, Sanal Ö. A novel mutation in leukocyte adhesion deficiency type II/CDGIIc. J Clin Immunol. 2014;34:1009–14.
Roos D, van Leeuwen K, Madkaikar M, Kambli PM, Gupta M, Mathews V, et al. Hematologically important mutations: leukocyte adhesion deficiency (second update). Blood Cells Mol Dis. 2023;102726
Fan Z, Ley K. Leukocyte arrest: biomechanics and molecular mechanisms of β2 integrin activation. Biorheology. 2015;52(5-6):353–77.
Kuijpers TW, Van Lier RA, Hamann D, de Boer M, Thung LY, Weening RS, et al. Leukocyte adhesion deficiency type 1 (LAD-1)/variant. A novel immunodeficiency syndrome characterized by dysfunctional beta2 integrins. J Clin Invest. 1997;100(7):1725–33.
Kambli PM, Bargir UA, Yadav RM, Gupta MR, Dalvi AD, Hule G, et al. Clinical and genetic spectrum of a large cohort of patients with leukocyte adhesion deficiency type 1 and 3: a multicentric study from India. Front Immunol. 2020;11:612703.
Bakhtiar S, Salzmann-Manrique E, Blok H-J, Eikema D-J, Hazelaar S, Ayas M, et al. Allogeneic hematopoietic stem cell transplantation in leukocyte adhesion deficiency type I and III. Blood Adv. 2021;5(1):262–73.
Robert P, Canault M, Farnarier C, Nurden A, Grosdidier C, Barlogis V, et al. A novel leukocyte adhesion deficiency III variant: kindlin-3 deficiency results in integrin- and nonintegrin-related defects in different steps of leukocyte adhesion. J Immunol. 2011;186(9):5273–83.
Goldstein B. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8.
Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. 2009;113(19):4740–6.
Kilic SS, Etzioni A. The clinical spectrum of leukocyte adhesion deficiency (LAD) III due to defective CalDAG-GEF1. J Clin Immunol. 2009;29(1):117–22.
Haskoloğlu ZŞ, Bal SK, Islamoğlu C, AKB B, Beşli D, Aytekin C, et al. Evaluation of clinical, immunological characteristics, treatment and follow-up of 14 patients with the diagnosis of leukocyte adhesion defect (type-I and type-III). Türkiye Çocuk Hast Derg/Turkish J Pediatr Dis. 2020;14:286–94.
Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, Ma YQ, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15(3):313–8.
Sabnis H, Kirpalani A, Horan J, McDowall A, Svensson L, Cooley A, et al. Leukocyte adhesion deficiency-III in an African-American patient. Pediatr Blood Cancer. 2010;55(1):180–2.
Elhasid R, Kilic SS, Ben-Arush M, Etzioni A, Rowe JM. Prompt recovery of recipient hematopoiesis after two consecutive haploidentical peripheral blood SCTs in a child with leukocyte adhesion defect III syndrome. Bone Marrow Transplant. 2010;45(2):413–4.
Crazzolara R, Maurer K, Schulze H, Zieger B, Zustin J, Schulz AS. A new mutation in the KINDLIN-3 gene ablates integrin-dependent leukocyte, platelet, and osteoclast function in a patient with leukocyte adhesion deficiency-III. Pediatr Blood Cancer. 2015;62(9):1677–9.
Palagano E, Slatter MA, Uva P, Menale C, Villa A, Abinun M, et al. Hematopoietic stem cell transplantation corrects osteopetrosis in a child carrying a novel homozygous mutation in the FERMT3 gene. Bone. 2017;97:126–9.
Meller J, Malinin NL, Panigrahi S, Kerr BA, Patil A, Ma Y, et al. Novel aspects of Kindlin-3 function in humans based on a new case of leukocyte adhesion deficiency III. J Thromb Haemost. 2012;10(7):1397–408.
Alon R, Aker M, Feigelson S, Sokolovsky-Eisenberg M, Staunton DE, Cinamon G, et al. A novel genetic leukocyte adhesion deficiency in subsecond triggering of integrin avidity by endothelial chemokines results in impaired leukocyte arrest on vascular endothelium under shear flow. Blood. 2003;101(11):4437–45.
Mory A, Feigelson SW, Yarali N, Kilic SS, Bayhan GI, Gershoni-Baruch R, et al. Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Blood. 2008;112(6):2591.
McDowall A, Svensson L, Stanley P, Patzak I, Chakravarty P, Howarth K, et al. Two mutations in the KINDLIN3 gene of a new leukocyte adhesion deficiency III patient reveal distinct effects on leukocyte function in vitro. Blood. 2010;115(23):4834–42.
Jurk K, Schulz AS, Kehrel BE, Rapple D, Schulze H, Mobest D, et al. Novel integrin-dependent platelet malfunction in siblings with leukocyte adhesion deficiency-III (LAD-III) caused by a point mutation in FERMT3. Thromb Haemost. 2010;103(5):1053–64.
Harris ES, Smith TL, Springett GM, Weyrich AS, Zimmerman GA. Leukocyte adhesion deficiency-I variant syndrome (LAD-Iv, LAD-III): molecular characterization of the defect in an index family. Am J Hematol. 2012;87(3):311.
Shahid S, Zaidi S, Ahmed S, Siddiqui S, Abid A, Malik S, et al. A novel nonsense mutation in FERMT3 causes LAD-III in a Pakistani family. Front Genet. 2019;10:360.
Essa MF, Elbashir E, Alroqi F, Mohammed R, Alsultan A. Successful hematopoietic stem cell transplant in leukocyte adhesion deficiency type III presenting primarily as malignant infantile osteopetrosis. Clin Immunol. 2020;213:108365.
Harris ES, Shigeoka AO, Li W, Adams RH, Prescott SM, McIntyre TM, et al. A novel syndrome of variant leukocyte adhesion deficiency involving defects in adhesion mediated by beta1 and beta2 integrins. Blood. 2001;97(3):767–76.
Stepensky PY, Wolach B, Gavrieli R, Rousso S, Ben Ami T, Goldman V, et al. Leukocyte adhesion deficiency type III: clinical features and treatment with stem cell transplantation. J Pediatr Hematol Oncol. 2015;37(4):264–8.
Suratannon N, Yeetong P, Srichomthong C, Amarinthnukrowh P, Chatchatee P, Sosothikul D, et al. Adaptive immune defects in a patient with leukocyte adhesion deficiency type III with a novel mutation in FERMT3. Pediatr Allergy Immunol. 2016;27(2):214–7.
Qureshi S, Mir F, Junejo S, Saleem K, Zaidi S, Naveed AB, et al. The spectrum of primary immunodeficiencies at a tertiary care hospital in Pakistan. World Allergy Organ J. 2020;13(7):100133.
Yahya AM, AlMulla AA, AlRufaye HJ, Al Dhaheri A, Elomami AS, Al-Hammadi S, et al. Case report: a case of leukocyte adhesion deficiency, type III presenting with impaired platelet function, lymphocytosis and granulocytosis. Front Pediatr. 2021;9:713921.
Schmidt S, Nakchbandi I, Ruppert R, Kawelke N, Hess MW, Pfaller K, et al. Kindlin-3–mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J Cell Biol. 2011;192(5):883–97.
Wolach B, Gavrieli R, Wolach O, Stauber T, Abuzaitoun O, Kuperman A, et al. Leucocyte adhesion deficiency-A multicentre national experience. Eur J Clin Invest. 2019;49(2):e13047.
Kinashi T, Aker M, Sokolovsky-Eisenberg M, Grabovsky V, Tanaka C, Shamri R, et al. LAD-III, a leukocyte adhesion deficiency syndrome associated with defective Rap1 activation and impaired stabilization of integrin bonds. Blood. 2004;103(3):1033–6.
Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, et al. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204(7):1571–82.
Slatkin M. Linkage disequilibrium—understanding the evolutionary past and mapping the medical future. Nat Rev Genet. 2008;9(6):477–85.
GnomAD 2022 [Available from: https://gnomad.broadinstitute.org/variant/11-64496517-G-T?dataset=gnomad_r2_1. .
Born G, Grayton HM, Langhorst H, Dudanova I, Rohlmann A, Woodward BW, et al. Genetic targeting of NRXN2 in mice unveils role in excitatory cortical synapse function and social behaviors. Front Synaptic Neurosci. 2015;7:3.
Ganesh A, Al-Zuhaibi SS, Bialasiewicz A, Ahmed S, Al-Tamemi S, E-Nour IB. Necrotizing Pseudomonas infection of the ocular adnexa in an infant with leukocyte adhesion defect. J Pediatr Ophthalmol Strabismus. 2007;44(4):199–200.
Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L. Loss of integrin α9β1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol. 2009;129(1):217–28.
Lee T-H, Seng S, Li H, Kennel SJ, Avraham HK, Avraham S. Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: role of α6β1 integrin in angiogenesis. J Biol Chem. 2006;281(52):40450–60.
Majorana A, Notarangelo LD, Savoldi E, Gastaldi G, Lozada-Nur F. Leukocyte adhesion deficiency in a child with severe oral involvement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(6):691–4.
Koivisto L, Heino J, Hakkinen L, Larjava H. Integrins in wound healing. Adv Wound Care. 2014;3(12):762–83.
Karow A, Baumann I, Niemeyer CM. Morphologic differential diagnosis of juvenile myelomonocytic leukemia—pitfalls apart from viral infection. J Pediatr Hematol Oncol. 2009;31(5):380.
Strauss A, Furlan I, Steinmann S, Buchholz B, Kremens B, Rossig C, et al. Unmistakable morphology? Infantile malignant osteopetrosis resembling juvenile myelomonocytic leukemia in infants. J Pediatr. 2015;167(2):486–8.
Hacettepe University Institute of Population Studies, 2018 Turkey Demographic and Health Survey. https://hips.hacettepe.edu.tr/en/2018_tdhs_analysis_and_report-262. Accessed 11 March 2023
Funding
The authors received no financial support for the research.
Author information
Authors and Affiliations
Contributions
ABK, IY, and DC wrote the manuscript. SA, AM, SSK, and IT reviewed the clinical and laboratory findings of the patients. IY and RG reviewed the genetic and radiological findings of the patients, respectively.
Corresponding author
Ethics declarations
Ethical Approval
The study design was in accordance with the Helsinki Declaration and was approved by the Hacettepe University Non-interventional Clinical Research Ethics Board (2021/12-56).
Consent to Participate
Informed consent was obtained from the patients and parents or legal guardians of the pediatric participants.
Consent for Publication
The participant has consented to the submission of the study to the journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key messages
• Leukocyte adhesion deficiency (LAD) type III leads to severe infections, such as sepsis, pneumonia, osteomyelitis, chorioretinitis, and cellulitis.
• Patients exhibit osteopetrosis-like bone findings and Glanzmann-type bleeding disorder.
• Leukocytosis, eosinophilia, and bone marrow findings may mimic pathologies such as JMML and MDS.
Supplementary Information
ESM 1.
(DOCX 196 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kahraman, A.B., Yaz, I., Gocmen, R. et al. Clinical and Osteopetrosis-Like Radiological Findings in Patients with Leukocyte Adhesion Deficiency Type III. J Clin Immunol 43, 1250–1258 (2023). https://doi.org/10.1007/s10875-023-01479-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-023-01479-7