Abstract
Cells of the innate and adaptive immune systems depend on proper actin dynamics to control cell behavior for effective immune responses. Dysregulated actin networks are known to play a pathogenic role in an increasing number of inborn errors of immunity. The WAVE regulatory complex (WRC) mediates branched actin polymerization, a process required for key cellular functions including migration, phagocytosis, vesicular transport, and immune synapse formation. Recent reports of pathogenic variants in NCKAP1L, a hematopoietically restricted gene encoding the HEM1 protein component of the WRC, defined a novel disease involving recurrent bacterial and viral infections, autoimmunity, and excessive inflammation (OMIM 141180). This review summarizes the diverse clinical presentations and immunological phenotypes observed in HEM1-deficient patients. In addition, we integrate the pathophysiological mechanisms described in current literature and highlight the outstanding questions for diagnosis and management of the HEM1 actin immunodysregulatory disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dynamic control of actin polymerization is crucial for cellular migration, adhesion, division, membrane transport, and spatiotemporal coordination of signals that govern metabolism and proliferation [1, 2]. Cells of the innate and adaptive immune systems depend on proper actin dynamics to control effective responses, which is highlighted by inborn errors of immunity (IEI) affecting actin networks, known as actinopathies (reviewed in [3,4,5,6,7]). Dysregulated actin networks cause recurrent infections often coupled with autoinflammation, autoimmunity, and atopic disease in IEIs such as Wiskott Aldrich syndrome, DOCK8 deficiency, and X-linked moesin-associated immune deficiency [3]. Recently, three reports investigating novel mutations in NCKAP1L, a hematopoietically restricted gene encoding the HEM1 protein component of the WAVE regulatory complex (WRC), established how improper actin networks lead to a clinical syndrome involving recurrent bacterial and viral infections and autoimmunity (OMIM 141180) [8,9,10]. One study showed how loss of the WRC, one of the two primary actin-related proteins 2/3 (Arp2/3) nucleation promoting factors (NPF), contributes to the complex picture of impaired specific immunity and concomitant nonspecific immune system hyperactivation [8]. In this review, we summarize the clinical manifestations and disease pathogenesis of HEM1 deficiency and propose future directions to improve therapy.
HEM1 and Branched Actin Polymerization
Actin networks are critical for kinetic processes in nearly all eukaryotic cells. Globular actin (G-actin) monomers are incorporated into either linear or branching polymers. In human immune cells, formin proteins polymerize G-actin into linear filamentous actin (F-actin) bundles to generate filopodia, which are protruding structures required for chemosensation. By contrast, branched actin networks are required for cell spreading and immune synapse formation, lamellipodia formation, endo- and exocytosis, and phagocytosis (Fig. 1). Additionally, a dense ring of branched actin surrounds the cytosol, creating a barrier at the cell cortex that regulates vesicle secretion [11,12,13]. Branched actin is polymerized by the ARP2/3 complex after stimulation by the Wiskott-Aldrich Syndrome protein (WASp) family of actin NPFs, which includes the WASp family verprolin homologous (WAVE) proteins. WAVE isoforms are the scaffold of the WRC, an obligate heteropentameric complex containing the additional subunits: hematopoietic protein 1/2 (HEM1/2), cytoplasmic FMR1 interacting protein 1/2 (CYFIP1/2), hematopoietic stem/progenitor cell protein 300 (HSPC300), and Abelson interactor 1/2 (ABI1/2). Differential RNA expression governs which isoform will be incorporated in the WRC [14]. WAVE2 is ubiquitously expressed and is the predominate form in hematopoietic cell lineages, while WAVE1 and WAVE3 expression is limited to neural tissues [15]. HEM1 expression is restricted to hematopoietic cells and its counterpart, HEM2, is expressed in all other tissues [16]. Tissue specificity of subunits suggests that composition of the WRC alters its regulation and function between different cell types.
Critical functions of branched actin in immune cells. (a) Sheets of branched actin protrude to form lamellipodia at the leading edge of migrating cells, guiding transport through extracellular matrices (ECM). (b) While the extracellular domains of integrin proteins bind to ligands in the ECM during migration or on neighboring cells during adhesion, their intracellular domains recruit components of focal adhesion complexes that bind to the cytoskeleton. Retrograde actin flow, where newly assembled actin pushes against the membrane and filaments elongate toward the cell center, generates tensile strength for integrin activation, focal adhesion maturation, and cell migration. (c) A ring of cortical branched actin below the cell membrane mechanically inhibits vesicle fusion and allows for coordinated exocytosis. (d) Branched actin is required for extracellular pathogen phagocytosis by innate immune cells. (e) At immune synapses between lymphocytes and antigen presenting cells, a ring of peripheral branched actin drives cell spreading across the activating surface
The HEM1/2 and CYFIP1/2 subunits of the WRC are structurally homologous and form a heterodimer with a flat, polybasic surface that facilitates plasma membrane association [1]. At the membrane, the WRC responds to a panoply of signals from phosphatidylinositol (3,4,5)-triphosphate, Abelson (ABL) and cyclin dependent kinase 5 (CDK5) kinases, and SH3-domain containing scaffolding proteins such as IRSp53 [1]. Importantly, small GTPases that cluster at the plasma membrane can also activate the WRC. Two distinct RAC1-binding sites on CYFIP1/2 and a recently discovered ARF1-binding site on HEM1/2 cooperatively activate the WRC [16, 17]. Combined, these WRC-activating signals initiate ARP2/3-mediated branched actin polymerization to generate lamellipodia at the cell’s leading edge (Fig. 1a) [2]. Tensile force generated by polarized F-actin polymerization allows for integrin activation and focal adhesion maturation, both of which are necessary for cellular adhesion and migration through extracellular matrices (Fig. 1b) [18, 19]. Additionally, branched actin is critical for morphological changes during phagocytosis (Fig. 1d) [20].
Beyond its structural necessity in immune cells, the actin cytoskeleton plays a central role in transmembrane signaling. A dense ring of cortical branched actin below the cell membrane is a barrier that mechanically inhibits nonspecific vesicle fusion, thereby allowing for coordinated exocytosis (Fig. 1c) [11, 13, 21]. ARP2/3-mediated actin polymerization drives cell spreading at immunological synapses and shapes the complex architectures that enable efficient antigen presentation and lymphocyte activation (Fig. 1e) [12, 22, 23]. Upon cellular stimulation, the WRC is recruited to the plasma membrane to communicate with central signaling molecules including phosphatidylinositol-3 kinases (PI3K), mitogen-activated protein kinases (MAPK), focal adhesion kinase (FAK), protein kinase B/AKT, and protein kinase C. These effectors subsequently govern activation, growth, proliferation, and survival [16, 24].
Features of HEM1 Deficiency
Genetics
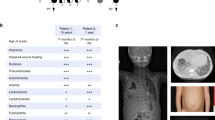
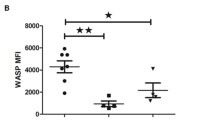
HEM1 deficiency was first described in 2019 and has been reported in nine individuals from seven kindreds [8,9,10, 25]. In the Genome Aggregation Database (gnomAD), the observed to expected ratio of loss-of-function (LoF) variants in NCKAP1L was 0.20 (90% CI 0.13–0.33), suggesting there is selection against HEM1 LoF [26]. All reported pathogenic variants in the 31-exon NCKAP1L gene on chromosome 12 (NM_005337.5) segregated with disease and followed autosomal recessive inheritance patterns (Fig. 2). Most are biallelic missense variants resulting in destabilizing amino acid substitutions: p.R129W in exon 4, p.V141F in exon 5, p.R258L in exon 7, and p.P359L and p.M371V in exon 11 [8, 9]. Two siblings carried compound heterozygous deleterious mutations encoding the p.P359L variant as well as a p.V519L variant in exon 16 [8]. None of the variants is present in the Exome Variant Server and only two variants (c.1076C > T [p.P359L] and c.1111A > G [p.M371V]) are observed in gnomAD, though there are no healthy homozygotes [26]. HEM1 protein modeling suggested that the affected amino acids, despite their locations on distant exons, cluster in a hotspot that is likely crucial for conformational stability [16]. These HEM1 amino acid substitutions destabilize the entire complex, causing subsequent degradation of all WRC subunits. Loss of all WRC components was also observed in cells from a patient who harbored a homozygous splice site mutation (c.2862 + 1G > A) resulting in exon 26 skipping [10]. By contrast, the p.M371V substitution drastically reduced WRC activation by the upstream GTPase ARF1, thus impairing WRC function without destabilizing the complex [8].
Clinical Features
HEM1-deficient patients typically present in the first year of life with dysregulated immune responses leading to a syndrome of immunodeficiency coupled with hyperinflammation, lymphoproliferation, and autoimmunity (Table 1). While the patient cohort reported by Salzer et al. displayed a predominantly autoimmune phenotype and those reported by Castro et al. had features consistent with hemophagocytic lymphohistiocytosis (HLH), the patients reported by Cook et al. had a mixed clinical picture, highlighting the overlapping features and commonalities. All patients suffer from recurrent infections such as otitis media, upper respiratory infections, pneumonia, and abscesses [8,9,10]. Respiratory infections commonly lead to chronic complications including bronchiectasis. Interstitial lung disease is also described without a clear etiology but may be related to viral infections or chronic inflammation [8, 27]. Other bacterial infections have included cellulitis, septic arthritis, and cholecystitis [8,9,10]. Reported viral infections have included disseminated herpes simplex virus, Epstein-Barr virus viremia, and gastroenteritis [8,9,10]. One patient has been described with lymphadenitis after Bacillus Calmette-Guerin vaccination, but this occurred after initiation of steroids [10]. Notably, siblings of patients from three of the seven kindreds died before 3 years of age from unknown causes or known infections, possibly because of HEM1 deficiency [8, 9].
Autoimmunity and hyperinflammation are commonly observed in HEM1-deficient patients. Patients may have systemic lupus erythematosus–like disease with positive anti-nuclear antibodies (ANA) and double-stranded (ds) DNA autoantibodies, and associated immune complex glomerulonephritis [8,9,10]. Episodes of fever can occur in conjunction with lymphadenopathy or hepatosplenomegaly. Autoimmune thrombocytopenia in one patient occurred after measles, mumps, and rubella vaccination [8]. Atopic disease in the form of food allergy, allergic rhinitis, and asthma occurs in more than half of the patient cohort, highlighting exaggerated atopic inflammatory responses against environmental allergens and irritants [8,9,10]. Three patients with features such as fever, splenomegaly, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia, and increased soluble IL-2 receptor met diagnostic criteria for hemophagocytic lymphohistiocytosis (HLH) [8, 10, 28, 29] and demonstrated transaminitis or sinusoidal dilatation on liver biopsy [8, 10]. One patient later developed hepatic fibrosis (data not shown). Lymph node biopsy from a different patient showed evidence of histiocytosis and emperipolesis suggesting Rosai-Dorfman disease [8].
Other clinical features include mild microcytic anemia and anisopoikilocytosis [10]. Interestingly, several dysmorphic and developmental syndromic features may be observed, such as intracerebral ventricular dilation, tricuspid valve deficiency, bicuspid aortic valve, ventricular septal defects, pectus carinatum, and poor dentition [8]. Failure to thrive was noted in two patients [8, 9].
Immunophenotype and Serologic Findings
Immunophenotypes in affected individuals have been variable (Table 1). Most patients (six out of nine) displayed increased total B cells [8,9,10]. Increased proportions of naïve B cells and B cell subsets associated with autoimmunity (innate-like memory B CD19+CD21loCD38lo and CD19+CD21−) were observed less consistently [9, 10]. Patients with severe autoimmune features display reduced proportions of naïve CD4+ and CD8+ T cell populations [8,9,10]. In contrast, subsets of CD4+ central memory (CM), effector memory (EM), and T effector memory RA+ (TEMRA) cells are frequently increased [9, 10]. Naïve CD8+ T cell populations were low, and no consistent pattern has been observed with CD8+ memory cells [8,9,10]. One investigation revealed increased exhaustion and senescence in patient CD4+ and CD8+ T cells, closely resembling the phenotype of DOCK8-deficient T cell [10, 30]. Two unrelated patients displayed persistently elevated proportions of αβ double-negative T (DNT) cells, an interesting comparison to the hallmark expansion of DNTs in autoimmune lymphoproliferative syndrome [8, 31, 32]. Natural killer (NK) cells were either normal or decreased across the cohort [8,9,10]. Subsequent investigations of immune cell population skewing were hampered by the use of immunosuppressive medications (see Therapeutic Perspectives). While immunophenotypes at disease presentation vary significantly, functional HEM1 is required for balanced lymphoid lineage hematopoiesis. This diversity of changes may be due to alterations of lymphocyte ontogeny and mature cell differentiation, as well as differences in genetics, epigenetic changes, and pathogen exposures.
Patients have variable immunoglobulin abnormalities. Elevated IgE is frequently observed, especially in those with asthma or atopic symptoms [8, 10]. More than half of the patients had elevated IgG levels without a clear relationship to autoimmune manifestations [8, 10]. One patient displayed selective IgA deficiency in the context of celiac disease while others displayed mildly elevated IgA levels [8, 10]. Impaired specific antibody production after vaccinations or EBV infection was observed in six patients [8, 10].
Proposed Pathogenic Mechanisms
Various mechanisms have been proposed to explain the array of abnormalities and clinical features observed in HEM1-deficient patients (Fig. 3), but gaps in our knowledge remain. Like mouse models of HEM1 deficiency, defects in neutrophil, dendritic cell, and macrophage migration and phagocytosis impair innate immune defenses, predisposing patients to bacterial infections [32,33,34,35]. Interestingly, myeloid-specific knockout of Hem1 in mice increased susceptibility to infection and morbidity upon influenza A and Streptococcus pneumoniae challenge [36]. Poor pathogen clearance, accumulation of debris, and excess cytokine production by alveolar macrophages may predispose patients to recurrent infections and long-term lung injury.
Because T cell immune synapses are highly dependent on ARP2/3-mediated F-actin polymerization [12, 22, 23, 37], HEM1-deficient T cells fail to properly integrate antigen signals and fully activate. This manifests as reduced CD25 and CD69 upregulation and poor proliferative responses in patient cells after TCR engagement in vitro [8, 9]. Similar defects in B cell immune synapses likely underlie poor antibody responses after vaccination or infection [9, 38]. Ultimately, insufficient innate and adaptive responses culminate in recurrent infections in HEM1-deficient patients.
Autoimmune features may result from impaired B cell receptor (BCR) signal strength during germinal center reactions leading to autoantibody production. During B cell development and BCR editing, defective actin polymerization at immune synapses results in weaker signals, allowing for survival of autoreactive B cells with inherently stronger signals [9, 39, 40]. Numerous Hem1-deficient mouse models demonstrated systemic autoimmunity and inflammation parallel to that seen in patients, including splenomegaly, immune complex lupus glomerulonephritis, elevated proinflammatory markers (IL-6, IFNγ, and MRP8/14), and autoreactive plasmablasts with elevated serum dsDNA autoantibodies [9, 41].
Prolonged pathogen exposure resulting from impaired adaptive immune cell activation is known to contribute to chronic inflammation and HLH development, potentially explaining the HLH-like phenotypes observed in patients [28, 42]. Despite failure to properly initiate adaptive responses, in vitro studies of activated patient CD8+ T cells revealed hypersecretion of cytotoxic cytokines, including perforin and granzymes A and B [8]. Knockdown of HEM1 in pan-T cells and subsequent reactivation resulted in increased levels of soluble granzymes, interferon gamma (IFNγ), IL-4, and IL-6, corresponding to increased serum levels of IFNγ and IL-6 observed in Hem1−/− mice [8, 9]. Multiple investigations found elevated surface CD107a levels after CD8 + T cell degranulation and lower cortical actin thickness, indicating that reduced integrity of the mechanical actin barrier facilitates vesicular hypersecretion [8, 10, 12, 33]. This draws an interesting comparison to the normal or elevated CD107a levels observed in the form of familial HLH in which granules lack functional perforin [28]. Diminished cortical actin barriers could also explain the observation that patient NK cells had higher basal degranulation rates [10]. An overall proinflammatory environment in secondary lymphoid organs, despite impaired specific lymphocyte activation, may explain the hepatomegaly, splenomegaly, and lymphadenopathy that was observed broadly across patient cohorts.
WRC and mTOR
Regulation of the cytoskeleton by mechanistic target of rapamycin (mTOR)–containing complex 2 (mTORC2) is well documented. It was initially shown that mTORC2 modulates cell morphology via PKCα signaling [43]. mTORC2 is activated by increased plasma membrane tension as part of a negative feedback system to limit actin-based cellular protrusions, thus strengthening the notion that mTORC2 is involved in mechanosensory cytoskeletal signaling cascades influencing cell shape and motility [44]. The investigation of HEM1-deficient patient cells and mouse models offers a unique insight into the relationship between mTORC2 and actin polymerization. Phosphorylation of AKT at serine 473, a specific target of the mTORC2 kinase complex, in response to various stimuli (CD3/28 TCR stimulation, IgM BCR stimulation, fibronectin, and ICAM-1) was diminished in T and B cells from patients [8, 9]. Impaired AKT activation and downstream dysregulation of Foxo1 substrates in Hem1-/- mouse B cells likely contributes to defects in proliferation and survival. Interestingly, a model of T cell–specific Wave2 knockout in mice produced the opposite effect; these mice displayed autoimmunity and cytokine hypersecretion similar to patients, but loss of WRC components resulted in enhanced mTORC1 and mTORC2 activity in mice [41]. These paradoxical findings suggest that distinct defects across multiple WRC-deficient hematopoietic lineages, rather than defects isolated to T cell lineages, interact to create a complex, systemic disease in humans. The exact mechanism of crosstalk between these two critical complexes is not fully understood; however, physical interaction between WRC and mTORC subunits was observed, suggesting direct modulation of kinase or actin polymerization activity [8, 41].
Biological Findings and Diagnosis
Initial clinical consideration of HEM1 deficiency may be challenging due to the clinical heterogeneity at presentation, as well as the phenotypic overlap with other known actinopathies and lymphoproliferative disorders. Findings of recurrent infections, poor responsiveness to vaccination, secondary lymphoid expansion, and serology suggestive of autoimmunity in the first year of life coupled with a negative genetic workup for autoimmune lymphoproliferative syndrome (ALPS) should prompt consideration of HEM1 deficiency as an alternative diagnosis [45]. Likewise, HEM1 deficiency should be ruled out in children presenting with hemophagocytic lymphohistiocytosis (HLH)–like autoinflammatory features [29]. The increasing availability of whole exome and genome sequencing in the diagnostic process should lower the threshold to initiate genetic testing in children with immune dysregulation. Initial investigations that should be considered, perhaps in collaboration with an immunology research laboratory, include immunoblotting to assess for WRC destabilization, though this test would have poor sensitivity in detecting variants that inhibit WRC activation without affecting protein levels. Supplementary tests may include T and B cell activation studies and neutrophil migration assays.
Therapeutic Perspectives
With the limited number of reported cases, proper therapy is still being defined. Corticosteroids and other immune suppressants such as sirolimus were clinically effective in controlling lymphoproliferation, autoimmunity, and auto-inflammatory symptoms. It is worth noting that using sirolimus is counterintuitive given in vitro data suggesting that HEM1 deficiency reduces mTORC activity [8, 41]. While this remains an area for investigation (see below), the clinical benefit of sirolimus in some patients is possibly attributable to reduced immune cell activation and proliferation in pro-inflammatory settings. Clinical responses to steroids were variable; one patient was successfully weaned off, but most required chronic steroids, azathioprine, or mycophenolate mofetil for long-term immune suppression. Rituximab was initiated in one patient during an acute exacerbation of nephrotic syndrome, leading to partial resolution of renal disease. Immunoglobulin replacement and prophylactic antimicrobials should be considered to prevent recurrent infections. Given the young age at presentation, severity of infections, systemic inflammatory complications (i.e., interstitial lung disease, glomerulonephritis, hepatic fibrosis), and dependence on immunosuppressants, hematopoietic stem cell transplant (HSCT) is a consideration. HSCT is a known treatment for other hematopoietic actinopathies such as WAS and DOCK8 deficiency and would likely rescue HEM1 in all relevant hematopoietic cell lineages. However, there are no data currently available for outcomes of HSCT in HEM1-deficient patients, including in some follow-up discussions regarding reported patients.
Areas for Investigation
Beyond blood, the HEM2-containing WRC has been broadly implicated in non-immunological diseases and is especially associated with tumor progression and metastasis in melanoma, non-small cell lung cancer, and breast cancer [46,47,48]. Additionally, neural tissues are highly dependent on cytoskeletal dynamics. Disruption of CYFIP2 and its interaction with Fragile X Mental Retardation Protein (FMRP) is known to cause a neurodevelopmental disorder characterized by intellectual disability and seizures [49]. Recently, WAVE2 was found to be directly modulated by leucine rich repeat kinase 2 (LRRK2) in microglia, establishing a potentially pathogenic role in development of Parkinson disease [50]. However, since HEM1 deficiency is the first reported WRC-related immunodeficiency, numerous mechanistic and clinical questions remain to be answered.
Immunoprecipitation and in vitro kinase studies showed that WAVE2 suppressed mTORC1 and mTORC2 activity [41]. However, data from patient cells reliably demonstrated reduced mTOR activity in the absence of WRC [8, 9]. Parsing out the intricate feedback loops between mTORC1 and mTORC2, and how the physical interaction of the WRC with mTORC impacts signaling cascades, will be necessary for understanding cellular defects in HEM1-deficient patients and the role of mTOR inhibitors in therapy. Furthermore, despite the instability of the WRC without each of its five subunits, some data suggest that HEM1 may exist in pools outside of the WRC. Determining the independent role of HEM1 could reveal novel pathways and additional therapeutic targets. A recent report demonstrated expression of an alternative NCKAP1L splice form in biliary epithelial cells promotes intrahepatic biliary tree morphogenesis [51]. These interesting findings should be validated, as extra-hematopoietic expression of NCKAP1L would have significant implications for disease pathogenesis and treatment.
As additional data become available from newly diagnosed patients, the full clinical picture of HEM1 deficiency will become clearer. Given the clinical heterogeneity at present, development of a clinical scoring system may assist in defining disease subtypes and determining genotype–phenotype correlations. Translational studies investigating immunomodulatory agents that limit inflammation and irreversible organ damage can drastically improve patient outcomes. Understanding the efficacy of HSCT will guide management, though the significant risks of rejection and graft-versus-host disease after HSCT make autologous gene-modified HSCT an appealing option [52, 53]. Ultimately, the best outcomes for patients with actin-related IEIs like HEM1 immunodysregulatory disorder will require early diagnosis and a precision medicine approach to management.
Data Availability
Not applicable as a review paper, but primary sources utilized can be made available upon request.
References
Chen B, Brinkmann K, Chen Z, Pak CW, Liao Y, Shi S, et al. The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell. 2014;156(1–2):195–207.
Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14(6):303–11.
Papa R, Penco F, Volpi S, Gattorno M. Actin remodeling defects leading to autoinflammation and immune dysregulation. Front Immunol. 2020;11:604206.
Sprenkeler EGG, Webbers SDS, Kuijpers TW. When actin is not actin’ like it should: a new category of distinct primary immunodeficiency disorders. J Innate Immun. 2021;13(1):3–25.
Chan AY, Torgerson TR. Primary immune regulatory disorders: a growing universe of immune dysregulation. Curr Opin Allergy Clin Immunol. 2020;20(6):582–90.
Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. The ever-increasing array of novel inborn errors of immunity: an interim update by the IUIS committee. J Clin Immunol. 2021;41(3):666–79.
Kamnev A, Lacouture C, Fusaro M, Dupré L. Molecular tuning of actin dynamics in leukocyte migration as revealed by immune-related actinopathies. Front Immunol. 2021;12:750537.
Cook SA, Comrie WA, Poli MC, Similuk M, Oler AJ, Faruqi AJ, et al. HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science. 2020;369(6500):202–7.
Salzer E, Zoghi S, Kiss MG, Kage F, Rashkova C, Stahnke S, et al. The cytoskeletal regulator HEM1 governs B cell development and prevents autoimmunity. Sci Immunol. 2020;5(49):eabc3979.
Castro CN, Rosenzwajg M, Carapito R, Shahrooei M, Konantz M, Khan A, et al. NCKAP1L defects lead to a novel syndrome combining immunodeficiency, lymphoproliferation, and hyperinflammation. J Exp Med 2020; 217(12):e20192275.
Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci. 2013;70(12):2099–121.
Ritter AT, Kapnick SM, Murugesan S, Schwartzberg PL, Griffiths GM, Lippincott-Schwartz J. Cortical actin recovery at the immunological synapse leads to termination of lytic granule secretion in cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2017;114(32):E6585–94.
Wollman R, Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat Cell Biol. 2012;14(12):1261–9.
Chen Z, Boren D, Padrick SB et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–8.
Suetsugu S, Miki H, Takenawa T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem Biophys Res Commun. 1999;260(1):296–302.
Rottner K, Stradal TEB, Chen B. WAVE regulatory complex. Curr Biol. 2021;31(10):R512–7.
Chen B, Chou HT, Brautigam CA, Xing W, Yang S, Henry L, et al. Rac1 GTPase activates the WAVE regulatory complex through two distinct binding sites. Elife. 2017;6:e29795.
Case LB, Waterman CM. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol. 2015;17(8):955–63.
Comrie WA, Babich A, Burkhardt JK. F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse. J Cell Biol. 2015;208(4):475–91.
Rougerie P, Miskolci V, Cox D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunol Rev. 2013;256(1):222–39.
Meunier FA, Gutiérrez LM. Captivating new roles of F-actin cortex in exocytosis and bulk endocytosis in neurosecretory cells. Trends Neurosci. 2016;39(9):605–13.
Dupré L, Boztug K, Pfajfer L. Actin dynamics at the T cell synapse as revealed by immune-related actinopathies. Front Cell Dev Biol. 2021;9:665519.
Hammer JA, Wang JC, Saeed M, Pedrosa AT. Origin, organization, dynamics, and function of actin and actomyosin networks at the T cell immunological synapse. Annu Rev Immunol. 2019;37:201–24.
Moujaber O, Stochaj U. The cytoskeleton as regulator of cell signaling pathways. Trends Biochem Sci. 2020;45(2):96–107.
Comrie WA, Poli MC, Cook SA, Similuk M, Oler AJ, Fauqi AJ, et al. Genetic immunodeficiency and autoimmune disease reveal distinct roles of Hem1 in the WAVE2 and mTORC2 complexes. bioRxiv. 2019.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–43.
Azadeh N, Limper AH, Carmona EM, Ryu JH. The role of infection in interstitial lung diseases: a review. Chest. 2017;152(4):842–52.
Brisse E, Wouters CH, Matthys P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol. 2016;174(2):203–17.
Ishii E. Hemophagocytic lymphohistiocytosis in children: pathogenesis and treatment. Front Pediatr. 2016;4:47.
Pillay BA, Avery DT, Smart JM, Cole T, Choo S, Chan D, et al. Hematopoietic stem cell transplant effectively rescues lymphocyte differentiation and function in DOCK8-deficient patients. JCI Insight. 2019;5:e127527.
Marlies A, Udo G, Juergen B, Bernd S, Herrmann M, Haas JP. The expanded double negative T cell populations of a patient with ALPS are not clonally related to CD4+ or to CD8+ T cells. Autoimmunity. England. 2007;40:299–301.
Park H, Staehling-Hampton K, Appleby MW, Brunkow ME, Habib T, Zhang Y, et al. A point mutation in the murine Hem1 gene reveals an essential role for Hematopoietic protein 1 in lymphopoiesis and innate immunity. J Exp Med. 2008;205(12):2899–913.
Chan MM, Wooden JM, Tsang M, Gilligan DM, Hirenallur-S DK, Finney GL, et al. Hematopoietic protein-1 regulates the actin membrane skeleton and membrane stability in murine erythrocytes. PLoS ONE. 2013;8(2):e54902.
Shao L, Chang J, Feng W, Wang X, Williamson EA, Li Y, et al. The Wave2 scaffold Hem-1 is required for transition of fetal liver hematopoiesis to bone marrow. Nat Commun. 2018;9(1):2377.
Leithner A, Eichner A, Müller J, Reversat A, Brown M, Schwarz J, et al. Diversified actin protrusions promote environmental exploration but are dispensable for locomotion of leukocytes. Nat Cell Biol. 2016;18(11):1253–9.
Suwankitwat N, Libby S, Liggitt HD, Avalos A, Ruddell A, Rosch JW, et al. The actin-regulatory protein Hem-1 is essential for alveolar macrophage development. J Exp Med. 2021;218(4):e20200472.
Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16(1):24–34.
Lin KB, Freeman SA, Zabetian S, Brugger H, Weber M, Lei V, et al. The rap GTPases regulate B cell morphology, immune-synapse formation, and signaling by particulate B cell receptor ligands. Immunity. 2008;28(1):75–87.
Kolhatkar NS, Brahmandam A, Thouvenel CD, Becker-Herman S, Jacobs HM, Schwartz MA, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome. J Exp Med. 2015;212(10):1663–77.
Li J, Yin W, Jing Y, Kang D, Yang L, Cheng J, et al. The coordination between B cell receptor signaling and the actin cytoskeleton during B cell activation. Front Immunol. 2018;9:3096.
Liu M, Zhang J, Pinder BD, Liu Q, Wang D, Yao H, et al. WAVE2 suppresses mTOR activation to maintain T cell homeostasis and prevent autoimmunity. Science. 2021;371(6536):eaaz4544.
Henter JI, Elinder G, Söder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78(11):2918–22.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–302.
Diz-Muñoz A, Thurley K, Chintamen S, Altschuler SJ, Wu LF, Fletcher DA, et al. Membrane tension acts through PLD2 and mTORC2 to limit actin network assembly during neutrophil migration. PLoS Biol. 2016;14(6):e1002474.
Price S, Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123(13):1989–99.
Swaminathan K, Campbell A, Papalazarou V, Jaber-Hijazi F, Nixon C, McGhee E, et al. The RAC1 target NCKAP1 plays a crucial role in the progression of Braf;Pten-driven melanoma in mice. J Invest Dermatol. 2021;141(3):628-37.e15.
Xiong Y, He L, Shay C, Lang L, Loveless J, Yu J, et al. Nck-associated protein 1 associates with HSP90 to drive metastasis in human non-small-cell lung cancer. J Exp Clin Cancer Res. 2019;38(1):122.
Teng Y, Qin H, Bahassan A, Bendzunas NG, Kennedy EJ, Cowell JK. The WASF3-NCKAP1-CYFIP1 complex is essential for breast cancer metastasis. Cancer Res. 2016;76(17):5133–42.
Begemann A, Sticht H, Begtrup A, Vitobello A, Faivre L, Banka S, et al. New insights into the clinical and molecular spectrum of the novel CYFIP2-related neurodevelopmental disorder and impairment of the WRC-mediated actin dynamics. Genet Med. 2021;23(3):543–54.
Kim KS, Marcogliese PC, Yang J, Callaghan SM, Resende V, Abdel-Messih E, et al. Regulation of myeloid cell phagocytosis by LRRK2 via WAVE2 complex stabilization is altered in Parkinson’s disease. Proc Natl Acad Sci U S A. 2018;115(22):E5164–73.
Ghaffari K, Pierce LX, Roufaeil M, Gibson I, Tae K, Sahoo S, et al. NCK-associated protein 1 like (nckap1l) minor splice variant regulates intrahepatic biliary network morphogenesis. PLoS Genet. 2021;17(3):e1009402.
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341(6148):1233151.
Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Díez IA, Dewey RA, et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med. 2010;363(20):1918–27.
Acknowledgements
We thank Drew Comrie for seminal studies on HEM1 deficiency. We thank Ryan Kissinger and Rose Gottschalk-Perry for their valuable illustrations.
Funding
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Author information
Authors and Affiliations
Contributions
SC, under mentorship of ML and AF, reviewed the available information about HEM1 and related diseases and prepared the manuscript. ML and AF reviewed the manuscript and provided intellectual content.
Corresponding authors
Ethics declarations
Ethics Approval/Consent to Participate/Consent for Publication
Not applicable as a review manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cook, S., Lenardo, M.J. & Freeman, A. . HEM1 Actin Immunodysregulatory Disorder: Genotypes, Phenotypes, and Future Directions. J Clin Immunol 42, 1583–1592 (2022). https://doi.org/10.1007/s10875-022-01327-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-022-01327-0