Abstract
Purpose
Specific granule deficiency (SGD) is a rare inborn error of immunity resulting from loss-of-function variants in CEBPE gene (encoding for transcription factor C/EBPε). Although this genetic etiology has been known for over two decades, only a few patients with CEBPE variant-proven SGD (type I) have been reported. Herein, we describe two siblings with a novel homozygous CEBPE deletion who were noted to have profound neutropenia on initial evaluation. We aimed to evaluate the immunohematological consequences of this novel variant, including profound neutropenia.
Methods
Light scatter characteristics of granulocytes were examined on various automated hematology analyzers. Phagocyte immunophenotype, reactive oxygen species generation, and Toll-like receptor (TLR) signaling were assessed using flow cytometry. Relative expression of genes encoding various granule proteins was studied using RT-PCR. Western blot analysis and luciferase reporter assay were performed to explore variant C/EBPε expression and function.
Results
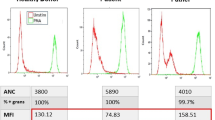
Severe infections occurred in both siblings. Analysis of granulocyte light scatter plots revealed automated hematology analyzers can provide anomalously low neutrophil counts due to abnormal neutrophil morphology. Neutrophils displayed absence/marked reduction of CD15/CD16 expression and overexpression (in a subset) of CD14/CD64. Three distinct populations of phagocytes with different oxidase activities were observed. Impaired shedding of CD62-ligand was noted on stimulation with TLR-4, TLR-2/6, and TLR-7/8 agonists. We demonstrated the variant C/EBPε to be functionally deficient.
Conclusion
Homozygous c.655_665del variant in CEBPE causes SGD. Anomalous automated neutrophil counts may be reported in patients with SGD type I. Aberrant TLR signaling might be an additional pathogenetic mechanism underlying immunodeficiency in SGD type I.







Similar content being viewed by others
Data Availability
Pertinent anonymized data are available in the Electronic Supplementary Material. The unanonymized datasets for this article are not publicly available due to concerns regarding participant/patient privacy/anonymity. Requests to access these datasets should be directed to the corresponding author.
Abbreviations
- BPI:
-
bactericidal permeability-increasing protein
- CAP18:
-
Cationic/cathelicidin antimicrobial protein–18 kDa
- CBCs:
-
complete blood counts
- CD62L:
-
CD62-ligand (L-selectin)
- C/EBP:
-
CCAAT/enhancer-binding protein
- ECP:
-
eosinophil cationic protein
- f-MLF:
-
N-formylmethionyl-leucyl-phenylalanine
- HEK:
-
human embryonic kidney
- IEI:
-
inborn error of immunity
- LCN2:
-
lipocalin-2
- LPS:
-
lipopolysaccharide
- LTF:
-
lactoferrin
- MBP:
-
major basic protein
- MRSA:
-
methicillin-resistant Staphylococcus aureus
- NADPH:
-
nicotinamide adenine dinucleotide phosphate hydrogen
- NBT:
-
nitroblue tetrazolium
- NC:
-
neutrophil collagenase
- P1 :
-
patient 1
- PMA:
-
phorbol 12-myristate 13-acetate
- RBC:
-
red blood cell
- ROS:
-
reactive oxygen species
- SDS-PAGE:
-
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
References
Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol. 2019;40(7):635–47.
Yin C, Heit B. Armed for destruction: formation, function and trafficking of neutrophil granules. Cell Tissue Res. 2018;371(3):455–71.
Bainton DF. Neutrophil granules. Br J Haematol. 1975;29(1):17–22.
Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273(1):11–28.
Wang W, Xia X, Mao L, Wang S. The CCAAT/enhancer-binding protein Family: its roles in MDSC expansion and function. Front Immunol. 2019;10:1804.
Larsen MT, Häger M, Glenthøj A, Asmar F, Clemmensen SN, Mora-Jensen H, et al. miRNA-130a regulates C/EBP-ε expression during granulopoiesis. Blood. 2014;123(7):1079–89.
Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365(3):561–75.
Bjerregaard MD, Jurlander J, Klausen P, Borregaard N, Cowland JB. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood. 2003;101(11):4322–32.
Wada T, Akagi T. Role of the leucine zipper domain of CCAAT/ enhancer binding protein-epsilon (C/EBPε) in neutrophil-specific granule deficiency. Crit Rev Immunol. 2016;36(4):349–58.
Lekstrom-Himes JA, Dorman SE, Kopar P, Holland SM, Gallin JI. Neutrophil-specific granule deficiency results from a novel mutation with loss of function of the transcription factor CCAAT/enhancer binding protein ε. J Exp Med. 1999;189(11):1847–52.
Gombart AF, Shiohara M, Kwok SH, Agematsu K, Komiyama A, Koeffler HP. Neutrophil-specific granule deficiency: homozygous recessive inheritance of a frameshift mutation in the gene encoding transcription factor CCAAT/enhancer binding protein–ε. Blood. 2001;97(9):2561–7.
Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1):24–64.
Aarts CEM, Downes K, Hoogendijk AJ, Sprenkeler EGG, Gazendam RP, Favier R, et al. Neutrophil specific granule and NETosis defects in gray platelet syndrome. Blood Adv. 2021;5(2):549–64.
Göös H, Fogarty CL, Sahu B, Plagnol V, Rajamäki K, Nurmi K, et al. Gain-of-function CEBPE mutation causes noncanonical autoinflammatory inflammasomopathy. J Allergy Clin Immunol. 2019;144(5):1364–76.
Witzel M, Petersheim D, Fan Y, Bahrami E, Racek T, Rohlfs M, et al. Chromatin-remodeling factor SMARCD2 regulates transcriptional networks controlling differentiation of neutrophil granulocytes. Nat Genet. 2017;49(5):742–52.
Yucel E, Karakus IS, Krolo A, Kiykim A, Heredia RJ, Tamay Z, et al. Novel frameshift autosomal recessive loss-of-function mutation in SMARCD2 encoding a chromatin remodeling factor mediates granulopoiesis. J Clin Immunol. 2021;41(1):59–65.
Hitoshi N, Ken-ichi Y, Jun-ichi M. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–9.
Wada T, Akagi T, Muraoka M, Toma T, Kaji K, Agematsu K, et al. A novel in-frame deletion in the leucine zipper domain of C/EBPε leads to neutrophil-specific granule deficiency. J Immunol. 2015;195(1):80–6.
Gery S, Gombart AF, Fung YK, Koeffler HP. C/EBPϵ interacts with retinoblastoma and E2F1 during granulopoiesis. Blood. 2004;103(3):828–35.
Serwas NK, Huemer J, Dieckmann R, Mejstrikova E, Garncarz W, Litzman J, et al. CEBPE-mutant specific granule deficiency correlates with aberrant granule organization and substantial proteome alterations in neutrophils. Front Immunol. 2018;9:588.
Shiohara M, Gombart AF, Sekiguchi Y, Hidaka E, Ito S, Yamazaki T, et al. Phenotypic and functional alterations of peripheral blood monocytes in neutrophil-specific granule deficiency. J Leukoc Biol. 2004;75(2):190–7.
Priam P, Krasteva V, Rousseau P, D’Angelo G, Gaboury L, Sauvageau G, et al. SMARCD2 subunit of SWI/SNF chromatin-remodeling complexes mediates granulopoiesis through a CEBPɛ dependent mechanism. Nat Genet. 2017;49(5):753–64.
Muraoka M, Akagi T, Ueda A, Wada T, Koeffler HP, Yokota T, et al. C/EBPε ΔRS derived from a neutrophil-specific granule deficiency patient interacts with HDAC1 and its dysfunction is restored by trichostatin A. Biochem Biophys Res Commun. 2019;516(1):293–9.
Yamanaka R, Barlow C, Lekstrom-Himes J, Castilla LH, Liu PP, Eckhaus M, et al. Impaired granulopoiesis, myelodysplasia, and early lethality in CCAAT/enhancer binding protein -deficient mice. Proc Natl Acad Sci U S A. 1997;94(24):13187–92.
Shigemura T, Yamazaki T, Shiohara M, Kobayashi N, Naganuma K, Koike K, et al. Clinical course in a patient with neutrophil-specific granule deficiency and rapid detection of neutrophil granules as a screening test. J Clin Immunol. 2014;34(7):780–3.
Rosenberg H, Gallin J. Neutrophil-specific granule deficiency includes eosinophils. Blood. 1993;82(1):268–73.
Ye Y, Carlsson G, Karlsson-Sjöberg JMT, Borregaard N, Modéer TU, Andersson ML, et al. The antimicrobial propeptide hCAP-18 plasma levels in neutropenia of various aetiologies: a prospective study. Sci Rep. 2015;5(1):11685.
Gallin J, Fletcher M, Seligmann B, Hoffstein S, Cehrs K, Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982;59(6):1317–29.
Leszcynska M, Patel B, Morrow M, Chamizo W, Tuite G, Berman DM, et al. Brain abscess as severe presentation of specific granule deficiency. Front Pediatr. 2020;8:117.
Ward PP, Mendoza-Meneses M, Park PW, Conneely OM. Stimulus-dependent impairment of the neutrophil oxidative burst response in lactoferrin-deficient mice. Am J Pathol. 2008;172(4):1019–29.
Verbeek W, Lekstrom-Himes J, Park DJ, Dang PM-C, Vuong PT, Kawano S, et al. Myeloid transcription factor C/EBPɛ is involved in the positive regulation of lactoferrin gene expression in neutrophils. Blood. 1999;94(9):3141–50.
von Bernuth H, Ku C-L, Rodriguez-Gallego C, Zhang S, Garty B-Z, Maródi L, et al. A fast procedure for the detection of defects in Toll-like receptor signaling. Pediatrics. 2006;118(6):2498–503.
Lekstrom-Himes J, Xanthopoulos KG. CCAAT/enhancer binding protein ɛ is critical for effective neutrophil-mediated response to inflammatory challenge. Blood. 1999;93(9):3096–105.
Kyme P, Thoennissen NH, Tseng CW, Thoennissen GB, Wolf AJ, Shimada K, et al. C/EBPε mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice. J Clin Invest. 2012;122(9):3316–29.
Strauss RG, Bove KE, Jones JF, Mauer AM, Fulginiti VA. An anomaly of neutrophil morphology with impaired function. N Engl J Med. 1974;290(9):478–84.
Boxer LA, Coates TD, Haak RA, Wolach JB, Hoffstein S, Baehner RL. Lactoferrin deficiency associated with altered granulocyte function. N Engl J Med. 1982;307(7):404–10.
Parmley RT, Tzeng DY, Baehner RL, Boxer LA. Abnormal distribution of complex carbohydrates in neutrophils of a patient with lactoferrin deficiency. Blood. 1983;62(3):538–48.
Ganz T, Metcalf JA, Gallin JI, Boxer LA, Lehrer RI. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and “specific” granule deficiency. J Clin Invest. 1988;82(2):552–6.
Lomax KJ, Gallin JI, Rotrosen D, Raphael GD, Kaliner MA, Benz EJ Jr, et al. Selective defect in myeloid cell lactoferrin gene expression in neutrophil specific granule deficiency. J Clin Invest. 1989;83(2):514–9.
Johnston JJ, Boxer LA, Berliner N. Correlation of messenger RNA levels with protein defects in specific granule deficiency. Blood. 1992;80(8):2088–91.
Khanna-Gupta A, Sun H, Zibello T, Lee HM, Dahl R, Boxer LA, Berliner N. Growth factor independence-1 (Gfi-1) plays a role in mediating specific granule deficiency (SGD) in a patient lacking a gene-inactivating mutation in the C/EBPepsilon gene. Blood. 2007;109(10):4181–90.
Murros J, Konttinen A. Recurrent attacks of abdominal pain and fever with familial segmentation arrest of granulocytes. Blood. 1974;43(6):871–4.
Repo H, Vuopio P, Leirisalo M, Jansson SE, Kosunen TU. Impaired neutrophil chemotaxis in Pelger-Huët anomaly. Clin Exp Immunol. 1979;36(2):326–33.
Pasanen AV, Ruutu P, Kosunen TU, Tenhunen R. Impaired heme synthesis in a family with Pelger-Huët anomaly, recurrent abdominal pain attacks and impaired neutrophil motility in vitro. Eur J Haematol. 1987;39(3):274–7.
Komiyama A, Morosawa H, Nakahata T, Miyagawa Y, Akabane T. Abnormal neutrophil maturation in a neutrophil defect with morphologic abnormality and impaired function. J Pediatr. 1979;94(1):19–25.
Ambruso DR, Sasada M, Nishiyama H, Kubo A, Komiyama A, Allen RH. Defective bactericidal activity and absence of specific granules in neutrophils from a patient with recurrent bacterial infections. J Clin Immunol. 1984;4(1):23–30.
Sakura T, Murakami H, Matsushima T, Tamura J, Sawamura M, Tsuchiya J. Ultrastructure of neutrophilic phagosome of autologous platelet in vivo in specific granule deficiency. Am J Hematol. 1993;43(2):149–50.
Tamura A, Agematsu K, Mori T, Kawai H, Kuratsuji T, Shimane M, et al. A marked decrease in defensin mRNA in the only case of congenital neutrophil-specific granule deficiency reported in Japan. Int J Hematol. 1994;59(2):137–42.
Breton-Gorius J, Mason DY, Buriot D, Vilde JL, Griscelli C. Lactoferrin deficiency as a consequence of a lack of specific granules in neutrophils from a patient with recurrent infections. Detection by immunoperoxidase staining for lactoferrin and cytochemical electron microscopy. Am J Pathol. 1980;99(2):413–28.
Stray-Pedersen A, Sorte HS, Samarakoon P, Gambin T, Chinn IK, Coban Akdemir ZH, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232–45.
Acknowledgements
The authors acknowledge the patients and their parents for agreeing to participate in the study. The authors also acknowledge the technical staff of Hematology laboratories in the Department of Pediatrics and Research Block-A, PGIMER, Chandigarh, for their kind help. We sincerely thank Dr. Deepak B Salunke, Department of Chemistry and Centre of Advanced Studies in Chemistry, Panjab University, Chandigarh, India, for providing TLR-2/6 and -7/8 agonists.
Author information
Authors and Affiliations
Contributions
AZB: inception of idea, writing of initial draft of manuscript, editing and revision of manuscript, patient evaluation and management, data acquisition and analysis, and review of literature.
AK, JD, IK, KA, GK: editing and revision of manuscript, performed experiments, and data acquisition and analysis.
TA, MM, TW: writing of initial draft of manuscript, editing and revision of manuscript, performed experiments, data acquisition and analysis, and review of literature.
DB: writing of initial draft of manuscript, editing and revision of manuscript, patient evaluation and management, data analysis, and review of literature.
DD: writing of initial draft of manuscript, editing and revision of manuscript, performed experiments, data acquisition and analysis, and review of literature.
MUSS, VP, AKJ, DS, JA, PB: editing and revision of manuscript, patient evaluation and management, data analysis, and review of literature.
HPK, HK, SS: editing and revision of manuscript, data analysis, and review of literature.
AR: editing and revision of manuscript, patient evaluation, data acquisition and analysis, review of literature, and overall supervision of manuscript preparation.
Corresponding authors
Ethics declarations
Ethics Approval
This study was conducted in accordance with the Helsinki Declaration and was approved by the Departmental Review Board, Department of Pediatrics, Advanced Pediatrics Center, PGIMER, Chandigarh. Volumes of blood obtained (by venipuncture) for the experiments were as follows – complete blood counts: 0.5–1.0 mL, peripheral blood immunophenotyping: 2 mL, dihydrorhodamine-123 and CD62-ligand shedding assays: 2 mL, Sanger sequencing and RT-PCR: 1 mL.
Consent to Participate/Publish
The patients and their parents consented to participate in the study and publish the manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data regarding serial complete blood counts and serial granulocyte scatter plots (on XN-1000 automated hematology analyzer) of our patient with SGD type II have not been published previously. Also, these details are not part of any other manuscript submitted for publication. However, the clinical profile of our patient with SGD type II is currently submitted as a separate case report/letter to the editor.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Banday, A.Z., Kaur, A., Akagi, T. et al. A Novel CEBPE Variant Causes Severe Infections and Profound Neutropenia. J Clin Immunol 42, 1434–1450 (2022). https://doi.org/10.1007/s10875-022-01304-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-022-01304-7