Abstract
Cartilage-hair hypoplasia is a syndromic immunodeficiency with short stature, chondrodysplasia, and variable degree of immune dysfunction. Patients with cartilage-hair hypoplasia are prone to recurrent respiratory tract infections, and the prevalence of bronchiectasis ranges from 29 to 52%. Pulmonary complications contribute significantly to the mortality; therefore, regular lung imaging is essential. However, the optimal schedule for repeated lung imaging remains unestablished. We determined the rate and correlates of progression of structural lung changes in a prospectively followed cohort of 16 patients with cartilage-hair hypoplasia. We analyzed clinical, laboratory, and pulmonary functional testing data and performed lung magnetic resonance imaging at a median interval of 6.8 years since previous imaging. Imaging findings remained identical or improved due to disappearance of inflammatory changes in all evaluated patients. Patients with subtle signs of bronchiectasis on imaging tended to have low immunoglobulin M levels, as well as suffered from pneumonia during the follow-up. In conclusion, our results suggest slow if any development of bronchiectasis in selected subjects with cartilage-hair hypoplasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cartilage-hair hypoplasia (CHH, MIM # 250250) is a syndromic immunodeficiency characterized by short stature, chondrodysplasia, and variable degree of immune dysfunction [1, 2]. CHH is caused by pathogenic variants in the RMRP gene, encoding the untranslated RNA component of the mitochondrial endoribonuclease RNase MRP [3]. The pathogenesis of immunodeficiency in CHH is complex and includes defective cell cycle, impaired telomere maintenance, and altered gene regulation [4,5,6]. Clinically, immune dysfunction in CHH leads to increased susceptibility to infections, mostly recurrent upper and lower respiratory tract infections, autoimmunity, and increased incidence of malignancy [7,8,9]. Bronchiectasis is common, with prevalence ranging from 29% in unselected patients and up to 52% in those with chronic respiratory symptoms [10, 11]. Pulmonary infections and bronchiectasis contribute significantly to the increased mortality [12]. Therefore, regular pulmonary imaging is essential, and bronchiectasis can reliably be followed up with lung magnetic resonance imaging (MRI), thus sparing patients from ionizing radiation [10]. However, the rate and determinants of progression of pulmonary changes and the optimal schedule for follow-up imaging in CHH remain unestablished.
Patients and Methods
We have followed a cohort of 16 patients with CHH, including all those patients for whom lung MRI had been performed in our previous study [10]. We aimed to (1) evaluate the progression of bronchiectasis at MRI, (2) analyze the pattern of respiratory infections, and (3) correlate clinical, laboratory, and pulmonary functional testing data with imaging findings. Informed consent was obtained from all patients and the study was approved by the Institutional Ethics Committee at the Helsinki University Hospital. The imaging equipment, protocol, and bronchiectasis scoring were identical to previously published; however, we used no contrast media [10].
Lung MRI was performed with a 1.5 T scanner (Achieva, Philips Medical System, Best, The Netherlands) with a same scanner and identical imaging protocol used in the previously published study, except for the contrast agent administration and the contrast-enhanced sequences. Approximate imaging time for MRI was 20 min. The protocol included the following sequences: (i) coronal breath-hold single-shot turbo spin echo (field of view (FOV) 315, time of repetition (TR) 786, time to echo (TE) 73.2, slice thickness 6 mm, spacing 4 mm), (ii) coronal and (iii) axial breath-hold 3-dimensional fast field echo (FOV 290, TR 3, TE 0.9, slice thickness 8 mm, spacing 4 mm), (iv) coronal balanced fast field echo (FOV 265, TR 3.4, TE 1.7, slice thickness 4 mm, spacing 2 mm), (v) axial fat-saturated T2-weighted (FOV 260, TR 4305, TE 60, slice thickness 6 mm, spacing 6.5), and (vi) axial and (vii) coronal respiratory- and cardiac-triggered T2-weighted turbo spin echo (FOV 280–327, TR 1500–1798, TE 90–100, slice thickness 5 and 8 mm), in (viii) inspirium and in (ix) expirium coronal balanced fast field echo (FOV 265, TR 3.4, TE 1.7, slice thickness 4 mm, spacing 2 mm).
All MRI studies were analyzed and scored by an experienced radiologist in random order in PACS workstation (Agfa Impax 6.5.2.2101). Scoring was performed using the modified Helbich (Bhalla) system [13]. Nine parameters were taken into account when evaluating high-resolution computed tomography (HRCT) and MRI images and a maximum possible score was 27 points. The score covered nine categories of changes, each scored from zero to three: (1) severity of bronchiectasis, (2) severity of peribronchial wall thickening, (3) extent of bronchiectasis, (4) extent of mucus plugging, (5) extent of sacculation or abscesses, (6) generation of bronchial division involved, (7) severity of bullae, (8) severity of emphysema, and (9) severity of collapse or consolidation. Score ≥ 7 was chosen as cutoff value for bronchiectasis.
We used Chi-square test to search for correlates between MRI score and clinical and laboratory variables. Statistical analyses were performed with IBM SPSS version 25 software.
Results
Table 1 describes clinical characteristics of the study patients. All patients were homozygous (n = 15) or compound heterozygous (n = 1, n.263G > T) for the n.71A > G RMRP mutation. Adult height in the majority of patients (15/16) represented growth between the 10 h and the 90th percentile on CHH-specific growth curves, while patient 10 demonstrated severe growth failure below the 10th percentile [14]. The cohort was diverse in their age (median 41 years, range 20–68 years, at the time of latest MRI) and in their clinical immunodeficiency phenotype (from asymptomatic to combined). The most common clinical symptoms of immunodeficiency in this cohort were recurrent rhinosinusitis, refractory mucocutaneous warts, as well as recurrent acute otitis media, which were reported in 11/16 (69%), 8/16 (50%), and 7/16 (44%) patients during lifetime, respectively. However, only two patients had ever received prophylactic antibiotics for recurrent respiratory tract infections, both in adulthood.
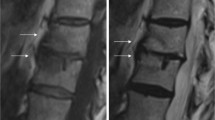
Table 2 demonstrates the results of pulmonary imaging and lung functional testing in the study patients. Repeated MRI was performed in 14/16 patients: at a median interval of 6.8 years (range 5.9–8.3 years). Patient 16 had deceased due to an unknown cause and for patient 13 logistic issues prevented imaging. MRI bronchiectasis scores remained identical to previous assessments in 11 patients and improved in three patients due to the disappearance of acute inflammatory changes. Figure 1 demonstrates the nonprogression of the structural changes in two patients. Of three patients with bronchiectasis (bronchiectasis score ≥ 7 points) detected already in their initial MRI, two did not undergo repeated imaging while the third cleared inflammatory changes (score dropped to <7). Three patients had also undergone repeated lung HRCT at the discretion of the treating physicians, and the results were compatible with repeated MRI, showing no progression of bronchiectasis. Lung diffusion capacity testing in three patients demonstrated normal results.
Pulmonary imaging of two study participants. Upper row: patient 6 with stable bronchiectasis (thick arrows) of the lower lobes; a the recent MRI (axial 3DFFE sequence) and 6 years earlier obtained, b baseline MRI (axial 3DFFE sequence), and c HRCT. Lower row: patient 2 with apical pleural thickening, parenchymal stranding and scarring (thin arrows), but no bronchiectasis. No progression of findings from baseline in HRCT or MRI imaging; d the recent MRI (axial T2 TSE sequence) and 6 years earlier obtained, e baseline MRI, and f HRCT
Two patients had acquired pneumonia during follow-up: patient 6 was treated at home, while patient 14 required hospitalization (Table 1). Both had some structural lung changes on initial imaging (6 and 4 points, respectively), which did not progress during follow-up. Our cohort was diverse in terms of other respiratory tract infections: while some patients remained infection-free during follow-up, others suffered from recurrent rhinosinusitis and otitis media. Five out of 16 participants (31%) had been previously diagnosed with asthma and four of them had been receiving regular inhaled corticosteroids starting from adolescence (n = 1) or adulthood (n = 3). The prevalence of asthma in our cohort was similar to the prevalence of 23% within a larger (n = 104) cohort of Finnish patients with CHH (p value in the Fisher exact test 0.53) [8]. None of the patients diagnosed with asthma developed novel or progressive previous lung changes on follow-up imaging. None of the patients received antibiotic prophylaxis during follow-up. Two patients received immunoglobulin (Ig) replacement therapy for the duration of follow-up. The indications for therapy had been recurrent infections, deficiency of one or more IgG subclasses, and specific antipolysaccharide antibody deficiency; none of the study participants had decreased total IgG. These two patients did not experience pneumonia or develop structural lung changes during follow-up. Neither did six other patients with zero bronchiectasis scores, who had not received Ig replacement.
The lymphocyte profile of study participants was variable, showing normal or low counts of measured lymphocyte subpopulations, but no correlation with lung changes (Table 3, Online Resource 1). The most consistent laboratory finding was the decreased number of recent thymic emigrants, B cells, and CD4+ T cells, detected in 9/16 (56%), 8/16 (50%), and 7/16 (44%) of patients, respectively. The unexpectedly high proportion of patients with normal total lymphocyte counts (14/16, 88%) is in contrast to the previously reported high prevalence of lymphopenia in Finnish CHH cohorts [1, 7, 15]. We could not perform the comparison of laboratory parameters between patients with and without bronchiectasis due to the absence of bronchiectasis on the repeated imaging.
There were no correlations between MRI bronchiectasis score and a range of clinical and laboratory parameters, including recurrent rhinosinusitis or otitis media, asthma, low counts of total lymphocytes, CD3+, CD4+, CD8+ T cells, CD19+ B cells, or low IgA levels (Online Resource 1). However, out of five patients with bronchiectasis MRI score higher than zero, three had low IgM levels and two had pneumonia during follow-up, compared to single patient with low IgM and no cases of pneumonia in patients with bronchiectasis score of zero (Χ2(4) = 9.9, p 0.042, and Χ2(4) = 9.6, p 0.047, for pneumonia and low IgM, respectively; Online Resource 1). These results should be interpreted with caution due to the limited sample size.
In our previous lung imaging study, additional 18 patients underwent HRCT pulmonary imaging, but not lung MRI. All 18 patients are alive at the time of writing. Among these patients, five were diagnosed with bronchiectasis: four with scores of 7 and one with score of 13 (Table 4). Follow-up lung imaging has been performed for four out of eleven patients with bronchiectasis score over zero (Table 4), while for others, follow-up imaging has been deemed clinically unnecessary. The follow-up imaging for these patients was performed outside the study protocol, which prevented the direct comparison of bronchiectasis scores. However, grossly evaluated, no (n = 3) or very subtle (n = 1) progression of bronchiectasis has been noticed on the follow-up imaging.
Discussion
We have previously reported the high prevalence of bronchiectasis in patients with CHH [10]. Since then, we have followed this reported cohort of patients to determine the rate and correlates of progression of pulmonary changes and the optimal schedule for follow-up imaging. We now describe the clinical and radiological outcomes with a median of 6.8 years of follow-up. The results suggest slow if any development of bronchiectasis in subjects with CHH. We provide evidence for the optimal schedule of follow-up lung imaging to be used by clinicians caring for similar patients.
The factors contributing to the development of bronchiectasis in some, but not all, patients with CHH remain unclear. Despite recurrent respiratory infections and the absence of antimicrobial prophylaxis, bronchiectasis did not develop in our study patients. Both patients who developed pneumonia during the follow-up had subtle structural lung changes that have not, however, progressed. Whether the preexisting pulmonary abnormalities predispose patients to lung infections, or whether the imaging findings and the infections independently reflect the more severe underlying immunodeficiency, remains to be confirmed in further studies.
Noteworthily, only one of our patients had been diagnosed with asthma in childhood, but had not received regular inhaled corticosteroids. Another four patients had been diagnosed with asthma in adolescence or adulthood and had all been treated with regular inhaled corticosteroids. It remains to be explored, whether appropriate management of asthma or asthma-like symptoms in childhood and/or adulthood may prevent the development of bronchiectasis.
We have previously reported higher T cell counts and higher IgG levels in CHH patients with bronchiectasis [10]. Patients in our follow-up cohort had a strikingly low prevalence of lymphopenia, which may underlie the milder course of immunodeficiency and possibly explain the absence of bronchiectasis. The finding of higher prevalence of low IgM levels in patients with subtle bronchiectasis changes is in concordance with reported correlations in patients with common variable immunodeficiency and bronchiectasis [16].
The rate of bronchiectasis development may be variable and influenced by various individual factors. The duration of our study (median 6.8 years of follow-up) may be insufficient to detect pulmonary structural changes. However, our results provide an approximate estimate of imaging schedule for mildly symptomatic patients. Based on our findings, routine pulmonary imaging can be scheduled infrequently, even at longer interval than the 6–8 years used in this study, in patients without bronchiectasis on initial imaging. However, the majority of subjects in our cohort with normal or nonprogressive findings on MRI did not experience pneumonia during follow-up. Therefore, patients with recurrent pneumonias probably warrant more frequent lung imaging. Also, data on patients with established bronchiectasis were limited in our study and no firm recommendations can be derived for the optimal time intervals between follow-up imaging studies.
One important limitation of our study is the genetic homogeneity of the Finnish CHH cohort and the associated restricted phenotype [17]. RMRP variants other than the Finnish founder variant n.71A > G can be associated with a more severe phenotype and therefore a different rate of development and/or progression of lung disease [18]. Further collaborative international effort is needed to expand our findings in a larger and genetically more heterogeneous CHH cohort.
The assessment of pulmonary function has been difficult in CHH, due to the absence of specific height-adjusted reference values in adults with short stature. In CHH, the height-related values may be normal even when lung function is diminished, whereas age-related values may be falsely low. For patients with common variable immunodeficiency, diffusion capacity testing does not discriminate patients with bronchiectasis from those without [19], and this may also be true for patients with CHH. In addition, some patients declined or were unable to perform functional testing. Therefore, the use of pulmonary functional testing in our cohort has been limited to three patients precluding interpretation.
HRCT follow-up imaging was not included in our study protocol due to concern about radiation burden; however, HRCT has been performed at the discretion of treating physicians in six patients, all showing none or very subtle progression of bronchiectasis. Although the direct comparison of bronchiectasis scores was impossible due to variability of imaging protocols, for the three patients with available MRI and HRCT follow-up imaging, the results were similar. Coupled with our previous findings [10], this suggests that while lung HRCT remains the gold standard for the initial evaluation of lung changes in patients with immunodeficiency, MRI can be implemented in the follow-up. In conclusion, we provide data on the lung imaging follow-up in patients with CHH. Several limitations should be applied when extrapolating these data to patients with more severe clinical course or established bronchiectasis. However, our results add to the limited knowledge on disease progression and proper management of patients with CHH.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Makitie O, Kaitila I. Cartilage-hair hypoplasia--clinical manifestations in 108 Finnish patients. Eur J Pediatr. 1993;152(3):211–7.
Kavadas FD, Giliani S, Gu Y, Mazzolari E, Bates A, Pegoiani E, et al. Variability of clinical and laboratory features among patients with ribonuclease mitochondrial RNA processing endoribonuclease gene mutations. J Allergy Clin Immunol. 2008;122(6):1178–84. https://doi.org/10.1016/j.jaci.2008.07.036.
Ridanpaa M, van Eenennaam H, Pelin K, Chadwick R, Johnson C, Yuan B, et al. Mutations in the RNA component of RNase MRP cause a pleiotropic human disease, cartilage-hair hypoplasia. Cell. 2001;104(2):195–203.
Kostjukovits S, Degerman S, Pekkinen M, Klemetti P, Landfors M, Roos G, et al. Decreased telomere length in children with cartilage-hair hypoplasia. J Med Genet. 2017;54(5):365–70. https://doi.org/10.1136/jmedgenet-2016-104279.
Rogler LE, Kosmyna B, Moskowitz D, Bebawee R, Rahimzadeh J, Kutchko K, et al. Small RNAs derived from lncRNA RNase MRP have gene-silencing activity relevant to human cartilage-hair hypoplasia. Hum Mol Genet. 2014;23(2):368–82. https://doi.org/10.1093/hmg/ddt427.
Vakkilainen S, Skoog T, Einarsdottir E, Middleton A, Pekkinen M, Öhman T, et al. The human long non-coding RNA gene RMRP has pleiotropic effects and regulates cell-cycle progression at G2. Sci Rep. 2019;9(1):13758. https://doi.org/10.1038/s41598-019-50334-6.
Kostjukovits S, Klemetti P, Valta H, Martelius T, Notarangelo LD, Seppanen M, et al. Analysis of clinical and immunologic phenotype in a large cohort of children and adults with cartilage-hair hypoplasia. The Journal of allergy and clinical immunology. 2017;140(2):612–4.e5. https://doi.org/10.1016/j.jaci.2017.02.016.
Vakkilainen S, Makitie R, Klemetti P, Valta H, Taskinen M, Husebye ES, et al. A wide Spectrum of autoimmune manifestations and other symptoms suggesting immune dysregulation in patients with cartilage-hair hypoplasia. Front Immunol. 2018;9:2468. https://doi.org/10.3389/fimmu.2018.02468.
Makitie O, Pukkala E, Teppo L, Kaitila I. Increased incidence of cancer in patients with cartilage-hair hypoplasia. J Pediatr. 1999;134(3):315–8.
Kostjukovits S, Klemetti P, Fohr A, Kajosaari M, Valta H, Taskinen M, et al. High prevalence of bronchiectasis in patients with cartilage-hair hypoplasia. J Allergy Clin Immunol. 2017;139(1):375–8. https://doi.org/10.1016/j.jaci.2016.07.023.
Toiviainen-Salo S, Kajosaari M, Piilonen A, Makitie O. Patients with cartilage-hair hypoplasia have an increased risk for bronchiectasis. J Pediatr. 2008;152(3):422–8. https://doi.org/10.1016/j.jpeds.2007.11.040.
Vakkilainen S, Taskinen M, Klemetti P, Pukkala E, Mäkitie O. A 30-year prospective follow-up study reveals risk factors for early death in cartilage-hair hypoplasia. Front Immunol. 2019;10:1581. https://doi.org/10.3389/fimmu.2019.01581.
Puderbach M, Eichinger M, Haeselbarth J, Ley S, Kopp-Schneider A, Tuengerthal S, et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: comparison to thin-section CT and chest x-ray. Investig Radiol. 2007;42(10):715–25. https://doi.org/10.1097/RLI.0b013e318074fd81.
Makitie O, Perheentupa J, Kaitila I. Growth in cartilage-hair hypoplasia. Pediatr Res. 1992;31(2):176–80. https://doi.org/10.1203/00006450-199202000-00018.
Makitie O, Kaitila I, Savilahti E. Susceptibility to infections and in vitro immune functions in cartilage-hair hypoplasia. Eur J Pediatr. 1998;157(10):816–20.
Ramzi N, Jamee M, Bakhtiyari M, Rafiemanesh H, Zainaldain H, Tavakol M, et al. Bronchiectasis in common variable immunodeficiency: a systematic review and meta-analysis. Pediatr Pulmonol. 2020;55(2):292–9. https://doi.org/10.1002/ppul.24599.
Ridanpaa M, Jain P, McKusick VA, Francomano CA, Kaitila I. The major mutation in the RMRP gene causing CHH among the Amish is the same as that found in most Finnish cases. American journal of medical genetics Part C, Seminars in medical genetics. 2003;121c(1):81–3. https://doi.org/10.1002/ajmg.c.20006.
Thiel CT, Mortier G, Kaitila I, Reis A, Rauch A. Type and level of RMRP functional impairment predicts phenotype in the cartilage hair hypoplasia-anauxetic dysplasia spectrum. Am J Hum Genet. 2007;81(3):519–29. https://doi.org/10.1086/521034.
Martínez García MA, de Rojas MD, Nauffal Manzur MD, Muñoz Pamplona MP, Compte Torrero L, Macián V, et al. Respiratory disorders in common variable immunodeficiency. Respir Med. 2001;95(3):191–5. https://doi.org/10.1053/rmed.2000.1020.
Acknowledgments
The authors are thankful to the research nurses Nea Boman, Päivi Turunen, and Ravna Broman for their help with arranging patient visits. The authors thank all patients for their participation.
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. This work is supported by the Sigrid Jusélius Foundation to [OM], the Academy of Finland to [OM], the Folkhälsan Research Foundation to [OM], the Novo Nordisk Foundation to [OM], the Helsinki University Hospital Research Funds to [OM], the Swedish Childhood Cancer Foundation to [OM], and the Foundation for Pediatric Research to [OM].
Author information
Authors and Affiliations
Contributions
STS, OM, and SV planned the study. PK, TM, and MS followed the patients. SV recruited patients, gathered and analyzed study data, and drafted the manuscript. All authors contributed to the writing of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Institutional Ethics Committee at the Helsinki University Hospital.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 91 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vakkilainen, S., Klemetti, P., Martelius, T. et al. Pulmonary Follow-Up Imaging in Cartilage-Hair Hypoplasia: a Prospective Cohort Study. J Clin Immunol 41, 1064–1071 (2021). https://doi.org/10.1007/s10875-021-01007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-021-01007-5