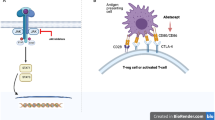
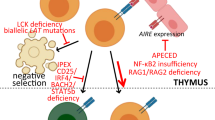
Abstract
IKAROS, encoded by IKZF1, is a zinc finger transcription factor and a critical regulator of hematopoiesis. Mutations in IKZF1 have been implicated in immune deficiency, autoimmunity, and malignancy in humans. Somatic IKZF1 loss-of-function mutations and deletions have been shown to increase predisposition to the development of B cell acute lymphoblastic leukemia (B-ALL) and associated with poor prognosis. In the last 4 years, germline heterozygous IKZF1 mutations have been reported in primary immune deficiency/inborn errors of immunity. These allelic variants, acting by either haploinsufficiency or dominant negative mechanisms affecting particular functions of IKAROS, are associated with common variable immunodeficiency, combined immunodeficiency, or primarily hematologic phenotypes in affected patients. In this review, we provide an overview of genetic, clinical, and immunological manifestations in patients with IKZF1 mutations, and the molecular and cellular mechanisms that contribute to their disease as a consequence of IKAROS dysfunction.



Similar content being viewed by others
References
Lo K, Landau NR, Smale ST. LyF-1, a transcriptional regulator that interacts with a novel class of promoters for lymphocyte-specific genes. Mol Cell Biol. 1991;11(10):5229–43. https://doi.org/10.1128/mcb.11.10.5229.
Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258(5083):808–12. https://doi.org/10.1126/science.1439790.
Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):143–56.
Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–99.
Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5(6):537–49. https://doi.org/10.1016/s1074-7613(00)80269-1.
Francis OL, Payne JL, Su RJ, Payne KJ. Regulator of myeloid differentiation and function: the secret life of Ikaros. World J Biol Chem. 2011;2(6):119–25. https://doi.org/10.4331/wjbc.v2.i6.119.
Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins SH, Trinchieri G, et al. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108(13):4025–34. https://doi.org/10.1182/blood-2006-03-007757.
Dijon M, Bardin F, Murati A, Batoz M, Chabannon C, Tonnelle C. The role of Ikaros in human erythroid differentiation. Blood. 2008;111(3):1138–46. https://doi.org/10.1182/blood-2007-07-098202.
Papathanasiou P, Perkins AC, Cobb BS, Ferrini R, Sridharan R, Hoyne GF, et al. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity. 2003;19(1):131–44.
Malinge S, Thiollier C, Chlon TM, Dore LC, Diebold L, Bluteau O, et al. Ikaros inhibits megakaryopoiesis through functional interaction with GATA-1 and NOTCH signaling. Blood. 2013;121(13):2440–51. https://doi.org/10.1182/blood-2012-08-450627.
Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16(8):2004–13. https://doi.org/10.1093/emboj/16.8.2004.
Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K, et al. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol. 1998;8(9):508–15.
Honma Y, Kiyosawa H, Mori T, Oguri A, Nikaido T, Kanazawa K, et al. Eos: a novel member of the Ikaros gene family expressed predominantly in the developing nervous system. FEBS Lett. 1999;447(1):76–80. https://doi.org/10.1016/s0014-5793(99)00265-3.
Perdomo J, Holmes M, Chong B, Crossley M. Eos and pegasus, two members of the Ikaros family of proteins with distinct DNA binding activities. J Biol Chem. 2000;275(49):38347–54. https://doi.org/10.1074/jbc.M005457200.
Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, et al. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 1998;12(6):782–96. https://doi.org/10.1101/gad.12.6.782.
Molnar A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol. 1994;14(12):8292–303. https://doi.org/10.1128/mcb.14.12.8292.
Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 1996;15(19):5358–69.
Georgopoulos K, Winandy S, Avitahl N. The role of the Ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol. 1997;15:155–76. https://doi.org/10.1146/annurev.immunol.15.1.155.
Sun L, Heerema N, Crotty L, Wu X, Navara C, Vassilev A, et al. Expression of dominant-negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1999;96(2):680–5. https://doi.org/10.1073/pnas.96.2.680.
Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–4. https://doi.org/10.1038/nature06866.
Nakase K, Ishimaru F, Avitahl N, Dansako H, Matsuo K, Fujii K, et al. Dominant negative isoform of the Ikaros gene in patients with adult B-cell acute lymphoblastic leukemia. Cancer Res. 2000;60(15):4062–5.
Meleshko AN, Movchan LV, Belevtsev MV, Savitskaja TV. Relative expression of different Ikaros isoforms in childhood acute leukemia. Blood Cells Mol Dis. 2008;41(3):278–83. https://doi.org/10.1016/j.bcmd.2008.06.006.
John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9–10):1272–8. https://doi.org/10.1016/j.molimm.2011.03.006.
Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, et al. Ikaros silences T-bet expression and interferon-gamma production during T helper 2 differentiation. J Biol Chem. 2010;285(4):2545–53. https://doi.org/10.1074/jbc.M109.038794.
Heizmann B, Sellars M, Macias-Garcia A, Chan S, Kastner P. Ikaros limits follicular B cell activation by regulating B cell receptor signaling pathways. Biochem Biophys Res Commun. 2016;470(3):714–20. https://doi.org/10.1016/j.bbrc.2016.01.060.
Dumortier A, Kirstetter P, Kastner P, Chan S. Ikaros regulates neutrophil differentiation. Blood. 2003;101(6):2219–26. https://doi.org/10.1182/blood-2002-05-1336.
Rao KN, Smuda C, Gregory GD, Min B, Brown MA. Ikaros limits basophil development by suppressing C/EBP-alpha expression. Blood. 2013;122(15):2572–81. https://doi.org/10.1182/blood-2013-04-494625.
Heizmann B, Kastner P, Chan S. The Ikaros family in lymphocyte development. Curr Opin Immunol. 2018;51:14–23. https://doi.org/10.1016/j.coi.2017.11.005.
Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80. https://doi.org/10.1056/NEJMoa0808253.
Kastner P, Dupuis A, Gaub MP, Herbrecht R, Lutz P, Chan S. Function of Ikaros as a tumor suppressor in B cell acute lymphoblastic leukemia. Am J Blood Res. 2013;3(1):1–13.
Goldman FD, Gurel Z, Al-Zubeidi D, Fried AJ, Icardi M, Song C, et al. Congenital pancytopenia and absence of B lymphocytes in a neonate with a mutation in the Ikaros gene. Pediatr Blood Cancer. 2012;58(4):591–7. https://doi.org/10.1002/pbc.23160.
Kuehn HS, Boisson B, Cunningham-Rundles C, Reichenbach J, Stray-Pedersen A, Gelfand EW, et al. Loss of B cells in patients with heterozygous mutations in IKAROS. N Engl J Med. 2016;374(11):1032–43. https://doi.org/10.1056/NEJMoa1512234.
Hoshino A, Okada S, Yoshida K, Nishida N, Okuno Y, Ueno H, et al. Abnormal hematopoiesis and autoimmunity in human subjects with germline IKZF1 mutations. J Allergy Clin Immunol. 2017;140(1):223–31. https://doi.org/10.1016/j.jaci.2016.09.029.
Bogaert DJ, Kuehn HS, Bonroy C, Calvo KR, Dehoorne J, Vanlander AV, et al. A novel IKAROS haploinsufficiency kindred with unexpectedly late and variable B-cell maturation defects. J Allergy Clin Immunol. 2018;141(1):432–5 e7. https://doi.org/10.1016/j.jaci.2017.08.019.
Dieudonne Y, Guffroy A, Vollmer O, Carapito R, Korganow AS. IKZF1 loss-of-function variant causes autoimmunity and severe familial antiphospholipid syndrome. J Clin Immunol. 2019;39(4):353–7. https://doi.org/10.1007/s10875-019-00643-2.
Van Nieuwenhove E, Garcia-Perez JE, Helsen C, Rodriguez PD, van Schouwenburg PA, Dooley J, et al. A kindred with mutant IKAROS and autoimmunity. J Allergy Clin Immunol. 2018;142(2):699–702 e12. https://doi.org/10.1016/j.jaci.2018.04.008.
Sriaroon P, Chang Y, Ujhazi B, Csomos K, Joshi HR, Zhou Q, et al. Familial immune thrombocytopenia associated with a novel variant in IKZF1. Front Pediatr. 2019;7:139. https://doi.org/10.3389/fped.2019.00139.
Groth DJ, Lakkaraja MM, Ferreira JO, Feuille EJ, Bassetti JA, Kaicker SM. Management of chronic immune thrombocytopenia and presumed autoimmune hepatitis in a child with IKAROS haploinsufficiency. J Clin Immunol. 2020;40(4):653–7. https://doi.org/10.1007/s10875-020-00781-y.
Eskandarian Z, Fliegauf M, Bulashevska A, Proietti M, Hague R, Smulski CR, et al. Assessing the functional relevance of variants in the IKAROS family zinc finger protein 1 (IKZF1) in a cohort of patients with primary immunodeficiency. Front Immunol. 2019;10:568. https://doi.org/10.3389/fimmu.2019.00568.
Chen QY, Wang XC, Wang WJ, Zhou QH, Liu DR, Wang Y. B-cell deficiency: a de novo IKZF1 patient and review of the literature. J Investig Allergol Clin Immunol. 2018;28(1):53–6. https://doi.org/10.18176/jiaci.0207.
Kuehn HS, Niemela JE, Stoddard J, Ciullini Mannurita S, Shahin T, Goel S, et al. Germline IKAROS dimerization haploinsufficiency causes hematologic cytopenias and malignancies. Blood. 2020. https://doi.org/10.1182/blood.2020007292.
Boutboul D, Kuehn HS, Van de Wyngaert Z, Niemela JE, Callebaut I, Stoddard J, et al. Dominant-negative IKZF1 mutations cause a T, B, and myeloid cell combined immunodeficiency. J Clin Invest. 2018;128(7):3071–87. https://doi.org/10.1172/JCI98164.
Nunes-Santos CJ, Kuehn HS, Rosenzweig SD. IKAROS family zinc finger 1-associated diseases in primary immunodeficiency patients. Immunol Allergy Clin N Am. 2020;40(3):461–70. https://doi.org/10.1016/j.iac.2020.04.004.
Iacobucci I, Storlazzi CT, Cilloni D, Lonetti A, Ottaviani E, Soverini S, et al. Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: on behalf of Gruppo Italiano Malattie Ematologiche dell'Adulto Acute Leukemia Working Party (GIMEMA AL WP). Blood. 2009;114(10):2159–67. https://doi.org/10.1182/blood-2008-08-173963.
Vairy S, Tran TH. IKZF1 alterations in acute lymphoblastic leukemia: the good, the bad and the ugly. Blood Rev. 2020:100677. https://doi.org/10.1016/j.blre.2020.100677.
Olsson L, Johansson B. Ikaros and leukaemia. Br J Haematol. 2015;169(4):479–91. https://doi.org/10.1111/bjh.13342.
Iacobucci I, Iraci N, Messina M, Lonetti A, Chiaretti S, Valli E, et al. IKAROS deletions dictate a unique gene expression signature in patients with adult B-cell acute lymphoblastic leukemia. PLoS One. 2012;7(7):e40934. https://doi.org/10.1371/journal.pone.0040934.
Martinelli G, Iacobucci I, Storlazzi CT, Vignetti M, Paoloni F, Cilloni D, et al. IKZF1 (Ikaros) deletions in BCR-ABL1-positive acute lymphoblastic leukemia are associated with short disease-free survival and high rate of cumulative incidence of relapse: a GIMEMA AL WP report. J Clin Oncol. 2009;27(31):5202–7. https://doi.org/10.1200/JCO.2008.21.6408.
van der Veer A, Zaliova M, Mottadelli F, De Lorenzo P, Te Kronnie G, Harrison CJ, et al. IKZF1 status as a prognostic feature in BCR-ABL1-positive childhood ALL. Blood. 2014;123(11):1691–8. https://doi.org/10.1182/blood-2013-06-509794.
Churchman ML, Qian M, Te Kronnie G, Zhang R, Yang W, Zhang H, et al. Germline genetic IKZF1 variation and predisposition to childhood acute lymphoblastic leukemia. Cancer Cell. 2018;33(5):937–48 e8. https://doi.org/10.1016/j.ccell.2018.03.021.
Cytlak U, Resteu A, Bogaert D, Kuehn HS, Altmann T, Gennery A, et al. Ikaros family zinc finger 1 regulates dendritic cell development and function in humans. Nat Commun. 2018;9(1):1239. https://doi.org/10.1038/s41467-018-02977-8.
Kellner ES, Krupski C, Kuehn HS, Rosenzweig SD, Yoshida N, Kojima S, et al. Allogeneic hematopoietic stem cell transplant outcomes for patients with dominant-negative IKFZ1/IKAROS mutations. J Allergy Clin Immunol. 2019;144:339–42. https://doi.org/10.1016/j.jaci.2019.03.025.
Acknowledgments
These studies were supported by the Intramural Research Program, NIH Clinical Center, US National Institutes of Health (NIH). The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Funding
This work was supported by the Intramural Research Program, NIH Clinical Center, US National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
HSK, CJNS, and SDR collected the data, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuehn, H.S., Nunes-Santos, C.J. & Rosenzweig, S.D. IKAROS-Associated Diseases in 2020: Genotypes, Phenotypes, and Outcomes in Primary Immune Deficiency/Inborn Errors of Immunity. J Clin Immunol 41, 1–10 (2021). https://doi.org/10.1007/s10875-020-00936-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-020-00936-x