Abstract
Structures of diasteromeric chalcone epoxides derivatives [3-(4-nitrophenyl)oxiran-2-yl)(phenyl)]-methanone (C15H11NO4), compound 1; (3,4-dimethoxy phenyl)-(3-(4-nitrophenyl)-oxiran-2-yl)methanone (C17H15NO6) compound 2 and [(3-(4-nitrophenyl)-oxiran-2-yl) phenyl-methanol] (C15H13NO4) compound 3 were established by spectral and X-ray diffraction studies. Compound 1 crystallizes in the monoclinic space group P21/c with unit cell parameters a = 10.3127(10), b = 10.45331(10), c = 12.9227(13) Å, β = 113.769(2)°, Z = 4. Compound 2 crystallizes in the triclinic space group P-1 with unit cell parameters a = 6.977(1), b = 8.259(1), c = 13.964(2) Å, α = 103.889(2), β = 91.151(2), γ = 100.780(2)°, Z = 2. Compound 3 crystallizes in the monoclinic space group P21 with unit cell parameters a = 6.708(1), b = 8.388(1), c = 12.407(2) Å, β = 103.322(3)°, Z = 2. The absolute configurations of 3 for the chiral centers are 1S, 2R, and 3R. In structures 1 and 2, the molecules in the crystal are linked by C–H···O interactions and van der Waals forces. Molecules 2 in the crystal are stacked in an anti-parallel fashion along the a-axis by π···π interactions which gives additional support to molecular packing stability. In structure 3, the molecules in the crystal are linked by both intra- and intermolecular hydrogen bonds O1–H···O2 and O1–H···O4, respectively. Adjacent molecules are interconnected by intermolecular weak C–H···O interactions.
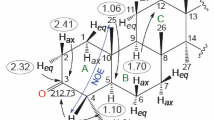
Graphical Abstract
Structures of three diasteromeric chalcone epoxides derivatives [3-(4-nitrophenyl)oxiran-2-yl)(phenyl)]-methanone 1; (3,4-dimethoxyphenyl)-(3-(4-nitrophenyl)-oxiran-2-yl)methanone 2 and [(3-(4-nitro phenyl)-oxiran-2-yl) phenyl-methanol] 3 were established by spectral and X-ray diffraction studies. The geometric parameters of the oxirane ring are in good agreement with those found in the literature and exist as trans isomer. The crystal packing in all three structures is stabilized by weak intermolecular C–H···O interactions. In structure 3, the molecules in the crystal are internally stabilized by both intra- and intermolecular O–H···O hydrogen bonds.






Similar content being viewed by others
References
Chetana BP, Mahajan SK, Suvarna AK (2009) J Pharma Sci Res 1:11
Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H (2003) Cancer Lett 201:133
Baba K, Taniguchi M, Nakata K (1998) Foods Food Ingred J Jpn 178:52
Hsieh HK, Lee TH, Wang JP, Wang JJ, Lin CN (1998) Pharm Res 15:39
Zhu XF, Xie BF, Zhou JM, Feng GK, Liu ZC, Wei XY, Zhang FX, Liu MF, Zeng YX (2005) Mol Pharmacol 67:1444
Anto RJ, Sukumaran K, Kuttan G, Rao MNA, Subbaraju V, Kuttan R (1995) Cancer Lett 97:33
Alcaraz MJ, Vicente A, Araico MA, Terencio MC, Ferrándiz ML (2004) Br J Pharmacol 142:1191
Ban HS, Suzuki K, Lim SS, Jung SH, Lee S, Ji J, Lee HS, Lee YS, Shin KH, Ohuchi K (2004) Biochem Pharmacol 67:1549
Madan B, Batra S, Ghosh B (2000) Mol Pharmacol 58:526
Araico A, Terencio MC, Alcaraz MJ, Domínguez JN, León C, Ferrándiz ML (2006) Life Sci 78:2911
Lee JH, Jung HS, Giang PM, Jin X, Lee S, Son PT, Lee D, Hong YS, Lee K, Lee JJ (2006) J Pharmacol Exp Ther 316:271
Therese M, Lantéri L, Fonbonne C, Huet J, Petit Ramel M (1987) Magn Reson Chem 25:216
Meth-Cohn O, Chen Y, Williams DJ (2000) Chem Commun 6:495
Armarego WLF, Perrin DD (1997) Purification of laboratory chemicals, 4th edn. Butterworth-Heinemann, Oxford
Go ML, Wu X, Liu XL (2005) Curr Med Chem 12:483
Bruker (2009) APEX2, SAINT and SADABS. Bruker AXS Inc., Madison
Sheldrick GM (2008) Acta Cryst A64:112
Brandenburg K (2006) DIAMOND. Version 3.1c. Crystal Impact GbR, Postfach 1251, D-53002 Bonn, Germany
Farrugia LJ (2012) J Appl Cryst 45:849
Nardelli M (1983) Comput Chem 7:95
Nardelli M (1995) J Appl Cryst 28:659
Baures PW, Eggleston DS, Flisak JR, Gombatz K, Lantos I, Mendelson W, Remich JJ (1990) Tetrahedron Lett 31:6501
Langer V, Li S, Lundquist K (2006) Acta Cryst C62:o625
Wasserman HH, Aubrey NE (1955) J Am Chem Soc 77:590
Matano Y (1994) J Chem Soc Perkin Trans 1:2703
Kumar A, Bhakuni V (1996) Tetrahedron Lett 37:4751
Acknowledgments
We are grateful for technical assistance from Rocío Patiño for IR spectra; Nieves Zavala, Héctor Ríos, Rubén Gaviño and Elizabeth Huerta for RMN spectra, Ruben A. Toscano and Simón Hernández for X-ray determinations; Luis Velasco for EM spectra. Financial support from DGAPA, UNAM Project PAPIIT IN216606 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Obregón-Mendoza, M.A., Escobedo-Martínez, C., Lozada, M.C. et al. Investigation of Three Diasteromeric Chalcone Epoxides Derivatives by NMR Spectroscopy and X-ray Crystallography. J Chem Crystallogr 44, 512–519 (2014). https://doi.org/10.1007/s10870-014-0544-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-014-0544-0