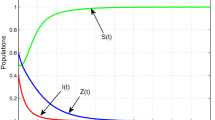
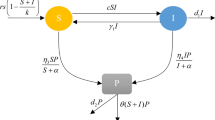
Abstract
In any ecosystem, chaotic situations may arise from equilibrium state for different reasons. To overcome these chaotic situations, sometimes the system itself exhibits some mechanisms of self-adaptability. In this paper, we explore an eco-epidemiological model consisting of three aquatic groups: phytoplankton, zooplankton, and marine free viruses. We assume that the phytoplankton population is infected by external free viruses and zooplankton get affected on consumption of infected phytoplankton; also, the infected phytoplankton do not compete for resources with the susceptible one. In addition, we model a mechanism by which zooplankton recognize and avoid infected phytoplankton, at least when susceptible phytoplankton are present. The zooplankton extinction chance increases on increasing the force of infection or decreasing the intensity of avoidance. Further, when the viral infection triggers chaotic dynamics, high zooplankton avoidance intensity can stabilize again the system. Interestingly, for high avoidance intensity, nutrient enrichment has a destabilizing effect on the system dynamics, which is in line with the paradox of enrichment. Global sensitivity analysis helps to identify the most significant parameters that reduce the infected phytoplankton in the system. Finally, we compare the dynamics of the system by allowing the infected phytoplankton also to share resources with the susceptible phytoplankton. A gradual increase of the virus replication factor turns the system dynamics from chaos to doubling state to limit cycle to stable state and the system finally settles down to the zooplankton-free equilibrium point. Moreover, on increasing the intensity of avoidance, the system shows a transcritical bifurcation from the zooplankton-free equilibrium to the coexistence steady state and remains stable thereafter.













Similar content being viewed by others
References
Vaulot, D.: Phytoplankton. In: Encyclopedia of Life Sciences, pp. 1–7. Macmillan Publishers Ltd. (2001)
Evans, C., Pond, D. W., Wilson, W. H.: Changes in Emiliania huxleyi fatty acid profiles during infection with E. huxleyi virus 86: physiological and ecological implications. Aquat. Microb. Ecol. 55, 219–228 (2009)
Gilg, I. C., et al.: Differential gene expression is tied to photochemical efficiency reduction in virally infected Emiliania huxleyi. Mar. Ecol. Prog. Ser. 555, 13–27 (2016)
Malitsky, S., et al.: Viral infection of the marine alga Emiliania huxleyi triggers lipidome remodeling and induces the production of highly saturated triacylglycerol. New Phytol. 210(1), 88–96 (2016)
Rosenwasser, S., et al.: Rewiring host lipid metabolism by large viruses determines the fate of Emiliania huxleyi, a bloom-forming alga in the ocean. Plant Cell tpc-114 (2014)
Suzuki, T., Yasuo, S.: Virus infection and lipid rafts. Biol. Pharm. Bull. 29 (8), 1538–1541 (2006)
Bratbak, G., Egge, J. K., Heldal, M.: Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar. Ecol. Prog. Ser. 93, 39–48 (1993)
Brussaard, C. P. D., et al.: Virus-like particles in a summer bloom of Emiliania huxleyi in the North Sea. Aquat. Microb. Ecol. 10, 105–113 (1996)
Castberg, T., et al.: Microbial population dynamics and diversity during a bloom of the marine coccolithophorid Emiliania huxleyi (Haptophyta). Mar. Ecol. Prog. Ser. 221, 39–46 (2001)
Nagasaki, K., et al.: Virus-like particles in Heterosiginu akashiwo (Raphidophyceae): a possible red-tide disintegration mechanism. Mar. Biol. 119(2), 307–312 (1994)
Jacquet, S., et al.: Flow cytometric analysis of an Emiliana huxleyi bloom terminated by viral infection. Aquat. Microb. Ecol. 27, 111–124 (2002)
Costamagna, A., et al.: A model for the operations to render epidemic-free a hog farm infected by the Aujeszky disease. Appl. Math. Nonlinear Sci. 1(1), 207–228 (2016)
Venturino, E.: Ecoepidemiology: a more comprehensive view of population interactions. Math. Model. Nat. Phenom. 11(1), 49–90 (2016)
Samanta, S., et al.: Effect of enrichment on plankton dynamics where phytoplankton can be infected from free viruses. Nonlinear Stud. 20(2), 223–236 (2013)
Bairagi, N., et al.: Virus replication factor may be a controlling agent for obtaining disease-free system in a multi-species eco-epidemiological system. J. Biol. Syst. 13(3), 245–259 (2005)
Evans, C., Wilson, W. H.: Preferential grazing of Oxyrrhis marina on virus infected Emiliania huxleyi. Limnol. Oceanogr. 53, 2035–2040 (2008)
Vermont, A., et al.: Virus infection of Emiliania huxleyi deters grazing by the copepod Acartia tonsa. J. Plankton Res. 38(5), 1194–1205 (2016)
Townsend, D. W., et al.: Blooms of the coccolithophore Emiliania huxleyi with respect to hydrography in the Gulf of Maine. Cont. Shelf Res. 14, 979–1000 (1994)
Wilson, W. H., et al.: Isolation of viruses responsible for the demise of an Emiliania huxleyi bloom in the English Channel. J. Mar. Biol. Assoc. U.K. 82(3), 369–377 (2002)
Evans, C.: The Influence of Marine Viruses on the Production of Dimethyl Sulphide (DMS) and Related Compounds from Emiliania Huxleyi. PhD Thesis, University of East Anglia (2005)
Evans, C., et al.: Viral infection of Emiliania huxleyi (Prymnesiophyceae) leads to elevated production of reactive oxygen species. J. Phycol. 42, 1040–1047 (2006)
Poulet, S. A., Ouellet, G.: The role of amino acids in the chemosensory swarming and feeding of marine copepods. J. Plankton Res. 4, 341–361 (1982)
Gill, C. W., Poulet, S. A.: Responses of copepods to dissolved free amino acids. Mar. Ecol. Prog. Ser. 43, 269–276 (1988)
Demott, W. R., Watson, M. D.: Remote detection of algae by copepods: responses to algal size, odors and motility. J. Plankton Res. 13, 1203–1222 (1991)
Steinke, M., Stefels, J., Stamhuis, E.: Dimethyl sulfide triggers search behavior in copepods. Limnol. Oceanogr. 51, 1925–1930 (2006)
Floge, S. A.: Virus infections of eukaryotic marine microbes, Electronic Theses and Dissertations. The University of Maine (2014)
Evans, C., et al.: The relative sinificance of viral lysis and microzooplankton grazing as pathways of dimethylsulfoniopropionate (DMSP) cleavage: an Emiliania huxleyi culture study. Limnol. Oceanogr. 52, 1036–1045 (2007)
Predators in the Plankton, Available at http://oceans.mit.edu/news/featured-stories/predators-in-the-plankton.html
Mukherjee, D.: Persistence in a prey-predator system with disease in the prey. J. Biol. Syst. 11(01), 101–112 (2003)
Venturino, E.: Epidemics in Predator-Prey Models: Disease in the Prey. In: Arino, O., Axelrod, D., Kimmel, M., Langlais, M. (eds.) Mathematical Population Dynamics: Analysis of Heterogeneity, vol. 1, pp 381–393 (1995)
Chattopadhyay, J., Arino, O.: A predator-prey model with disease in the prey. Nonlinear Anal. 36, 747–766 (1999)
Hethcote, H. W., et al.: A predator-prey model with infected prey. Theor. Popul. Biol. 66(3), 259–268 (2004)
Beretta, E., Kuang, Y.: Modeling and analysis of a marine bacteriophage infection. Math. Biosci. 149, 57–76 (1998)
Siekmann, I., Malchow, H., Venturino, E.: An extension of the Beretta-Kuang model of viral diseases. Math. Biosci. Eng. 5, 549–565 (2008)
Hilker, F. M., et al.: Oscillations and waves in a virally infected plankton system Part II: Transition from lysogeny to lysis. Ecol. Compl. 3, 200–208 (2006)
Bhattacharyya, S., Bhattacharya, D. K.: Pest control through viral disease: mathematical modeling and analysis. J. Theor. Biol. 238(1), 177–197 (2006)
Beltrami, E., Carroll, T. O.: Modeling the role of viral disease in recurrent phytoplankton blooms. J. Math. Biol. 32, 857–863 (1994)
Gakkhar, S., Negi, K.: A mathematical model for viral infection in toxin producing phytoplankton and zooplankton system. Appl. Math. Comp. 179, 301–313 (2006)
Chattopadhyay, J., Pal, S.: Viral infectionon phytoplankton-zooplankton system: a mathematical model. Ecol. Model. 151, 15–28 (2002)
Rhodes, C., Truscott, J., Martin, A.: Viral infection as a regulator of oceanic phytoplankton populations. J. Mar. Syst. 74, 216–226 (2008)
Singh, B. K., Chattopadhyay, J., Sinha, S.: The role of virus infection in a simple phytoplankton-zooplankton system. J. Theor. Biol. 231, 153–166 (2004)
Rhodes, C. J., Martin, A. P.: The influence of viral infection on a plankton ecosystem undergoing nutrient enrichment. J. Theor. Biol. 265(3), 225–237 (2010)
May, R.M.: Chaos and the dynamics of biological populations. Proc. R. Soc. Lond. 413, 27–44 (1987)
Godfray, H.C.J., Grenfell, B.T.: The continuing quest for chaos. Trends Ecol. Evol. 8, 43–44 (1993)
Hastings, A., et al.: Chaos in ecology: is mother nature a strange attractor?. Annu. Rev. Ecol. Syst. 24, 1–33 (1993)
Perry, J.N., Woiwod, I.P., Hanski, I.: Using response-surface methodology to detect chaos in ecological time series. Oikos 68, 329–339 (1993)
Jørgensen, S.E.: The growth rate of zooplankton at the edge of chaos: ecological models. J. Theor. Biol. 175, 13–21 (1995)
Hastings, A., Powell, T.: Chaos in three-species food chain. Ecology 72, 896–903 (1991)
Chattopadhyay, J., Sarkar, R.R.: Chaos to order: preliminary experiments with a population dynamics models of three trophic levels. Ecol. Model. 163, 45–50 (2003)
Peters, R.H.: The Ecological Implications of Body Size. Cambridge University Press, Cambridge (1983)
Mandal, S., et al.: Order to chaos and vice versa in an aquatic ecosystem. Ecol. Model. 197, 498–504 (2006)
Chakraborty, S., et al.: The role of avoidance by zooplankton for survival and dominance of toxic phytoplankton. Ecol. Compl. 11, 144–153 (2012)
Holmes, J. C., Bethel, W. M.: Modification of intermediate host behavior by parasites. In: Canning, E.V., Wright, C.A. (eds.) Behavioral Aspects of Parasite Transmission, vol. 51, pp 123–149. Suppl. I to Zool. F. Linnean Soc. (1972)
Lafferty, K. D.: Foraging on prey that are modified by parasites. Am. Nat. 140, 854–867 (1992)
Hamilton, W. D., Axelrod, R., Tanese, R.: Sexual reproduction as an adaptation to resist parasites: a review. Proc. Natl Acad. Sci. USA 87, 3566–3573 (1990)
Uhlig, G., Sahling, G.: Long-term studies on Noctiluca scintillans in the German Bight population dynamics and red tide phenomena 1968–1988. Neth. J. Sea Res. 25, 101–112 (1992)
Lakshmikantham, V., Leela, S., Martynyuk, A. A.: Stability analysis of nonlinear systems. Marcel Dekker, Inc, New York/Basel (1989)
Smith, H.L.: The Rosenzweig-MacArthur predator-prey model. https://math.la.asu.edu/halsmith/Rosenzweig.pdf
Li, Y., Muldowney, J. S.: On Bendixson’s criterion. J. Diff. Eqn. 106, 27–39 (1993)
Marino, S., et al.: A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254(1), 178–196 (2008)
Rosenzweig, M. L.: Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science 171, 385 (1971)
Linhart, S. B., et al.: Avoidance of prey by captive coyotes punished with electric shock. In: Proceedings of the Vertebrate Pest Conference, vol. 7, pp. 302–330 (1976)
Lebedeva, L.P.: A model of the latitudinal distribution of the numbers of species of phytoplankton in the sea. J. Cons. Int. Explor. Mer. 34, 341–350 (1972)
Jørgensen, S.E., et al.: Improved calibration of a eutrophication model by use of the size variation due to succession. Ecol. Model. 153, 269–277 (2002)
Odum, H.T.: Self organization, transformity, and information. Science 242, 1132–1139 (1988)
Kauffman, S.A.: Anti-chaos and adaptation. Sci. Am. 265(2), 78–84 (1991)
Acknowledgments
The authors are grateful to the anonymous referees for their careful reading, valuable comments, and helpful suggestions, which have contributed to improve the presentation of this work significantly. The authors are grateful to Prof. Guido Badino, DBIOS, University of Turin, Italy, for his valuable suggestions.
Funding
The research work of Saswati Biswas is supported by Council of Scientific and Industrial Research, Government of India, New Delhi in the form of Senior Research Fellowship (Ref. No. 20/12/2015(ii)EU-V). Pankaj Kumar Tiwari is thankful to University Grants Commissions, New Delhi, India for providing financial support in form of D. S. Kothari post-doctoral fellowship (No. F.4-2/2006 (BSR)/MA/17-18/0021). Research of Samares Pal is supported by DST-FIST programme of University of Kalyani (474 (Sanc.)/ST/P/S & T/16 G-22/2018). Ezio Venturino has been partially supported by the project “Metodi numerici e computazionali per le scienze applicate” of the Dipartimento di Matematica “Giuseppe Peano”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Member of the INdAM research group GNCS.
Rights and permissions
About this article
Cite this article
Biswas, S., Tiwari, P.K., Bona, F. et al. Modeling the avoidance behavior of zooplankton on phytoplankton infected by free viruses. J Biol Phys 46, 1–31 (2020). https://doi.org/10.1007/s10867-020-09538-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-020-09538-5