Abstract
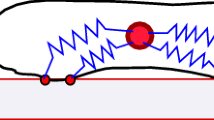
During cell migration, forces applied to a cell from its environment influence the motion. When the cell is placed on a substrate, such a force is provided by the cell-substrate adhesion. Modulation of adhesivity, often performed by the modulation of the substrate stiffness, tends to cause common responses for cell spreading, cell speed, persistence, and random motility coefficient. Although the reasons for the response of cell spreading and cell speed have been suggested, other responses are not well understood. In this study, we develop a simple toy model for cell migration driven by the relation of two forces: the adhesive force and the plasma membrane tension. The simplicity of the model allows us to perform the calculation not only numerically but also analytically, and the analysis provides formulas directly relating the adhesivity to cell spreading, persistence, and the random motility coefficient. Accordingly, the results offer a unified picture on the causal relations between those multiple cellular responses. In addition, cellular properties that would influence the migratory behavior are suggested.







Similar content being viewed by others
References
Oakes, P.W., Patel, D.C., Morin, N.A., Zitterbart, D.P., Fabry, B, Reichner, J.S., Tang, J.X.: Neutrophil morphology and migration are affected by substrate elasticity. Blood 114, 1387–1395 (2009)
Jannat, R.A., Dembo, M., Hammer, D.A.: Traction forces of neutrophils migrating on compliant substrates. Biophys. J. 101, 575–584 (2011)
Lo, C.M., Wang, H.B., Dembo, M, Wang, Y.L.: Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 (2000)
Wang, H.B., Dembo, M., Wang, Y.L.: Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 279, C1345–C1350 (2000)
Paszek, M.J., Zahir, N, Johnson, K.R., Lakins, J.N., Rozenberg, G.I., Gefen, A., Reinhart-King, C.A., Margulies, S.S., Dembo, M., Boettiger, D., Hammer, D.A., Weaver, V.M.: Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005)
Guo, W.H., Frey, M.T., Burnham, N.A., Wang, Y.L.: Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 90, 2213–2220 (2006)
Ghosh, K, Pan, Z, Guan, E, Ge, S, Liu, Y, Nakamura, T, Ren, X.D., Rafailovich, M, Clark, R.A.F.: Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 28, 671–679 (2007)
Engler, A.J., Griffin, M.A., Sen, S., Bönnemann, C. G., Sweeney, H.L., Discher, D.E.: Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, 877–887 (2004)
Mitrossilis, D., Fouchard, J., Guiroy, A., Desprat, N., Rodriguez, N., Fabry, B., Asnacios, A.: Single-cell response to stiffness exhibits muscle-like behavior. Proc. Natl. Acad. Sci. USA 106, 18243–18248 (2009)
Chan, C.E., Odde, D.J.: Traction dynamics of filopodia on compliant substrates. Science 322, 1687–1691 (2008)
Yeung, T., Georges, P.C., Flanagan, L.A., Marg, B., Ortiz, M., Funaki, M., Zahir, N., Ming, W., Weaver, V., Janmey, P.A.: Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 60, 24–34 (2005)
Stroka, K.M., Aranda-Espinoza, H.: Neutrophils display biphasic relationship between migration and substrate stiffness. Cell Motil. Cytoskeleton 66, 328–341 (2009)
Jannat, R.A., Robbins, G.P., Ricart, B.G., Dembo, M., Hammer, D.A.: Neutrophil adhesion and chemotaxis depend on substrate mechanics. J. Phys.: Condens. Matter 22, 194117 (2010)
Pelham, R.J. Jr, Wang, Y.L.: Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94, 13661–13665 (1997)
Jiang, G., Huang, A.H., Cai, Y., Tanase, M., Sheetz, M.P.: Rigidity sensing at the leading edge through α νβ 3 integrins and RPTPα. Biophys. J. 90, 1804–1809 (2006)
Solon, J., Levental, I., Sengupta, K., Georges, P.C., Janmey, P.A.: Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 93, 4453–4461 (2007)
Tzvetkova-Chevolleau, T., Steṕhanou, A., Fuard, D., Ohayon, J., Schiavone, P., Tracqui, P.: The motility of normal and cancer cells in response to the combined influence of the substrate rigidity and anisotropic microstructure. Biomaterials 29, 1541–1551 (2008)
Lovett, D.B., Shekhar, N., Nickerson, J.A., Roux, K.J., Lele, T.P.: Modulation of nuclear shape by substrate rigidity. Cell Mol. Bioeng. 6, 230–238 (2013)
Kim, D.H., Wirtz, D.: Focal adhesion size uniquely predicts cell migration. FASEB J. 27, 1351–1361 (2013)
Oakes, P.W., Bidone, T.C., Beckham, Y., Skeeters, A.V., Ramirez-San Juan, G.R., Winter, S.P., Voth, G.A., Gardel, M.L.: Lamellipodium is a myosin-independent mechanosensor. Proc. Natl. Acad. Sci. USA 115, 2646–2651 (2018)
Wong, J.Y., Velasco, W.J., Rajagopalan, A., Pham, Q.: Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir 19, 1908–1913 (2003)
Engler, A., Bacakova, L., Newman, C., Hategan, A., Griffin, M., Discher, D.: Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 86, 617–628 (2004)
Peyton, S.R., Putnam, A.J.: Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell Physiol. 204, 198–209 (2005)
Peyton, S.R., Raub, C.B., Keschrumrus, V.P., Putnam, A.J.: The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27, 4881–4893 (2006)
Engler, A.J., Carag-Krieger, C., Johnson, C.P., Raab, M., Tang, H.Y., Speicher, D.W., Sanger, J.W., Sanger, J.M., Discher, D.E.: Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 121, 3794–3802 (2008)
Califano, J.P., Reinhart-King, C.A.: A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell Mol. Bioeng. 1, 122–132 (2008)
Reinhart-King, C.A., Dembo, M., Hammer, D.A.: Cell-cell mechanical communication through compliant substrates. Biophys. J. 95, 6044–6051 (2008)
Discher, D.E., Janmey, P., Wang, Y.L.: Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005)
Nemir, S., West, J.L.: Synthetic materials in the study of cell response to substrate rigidity. Ann. Biomed. Eng. 38, 2–20 (2010)
Gray, D.S., Tien, J., Chen, C.S.: Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’ modulus. J. Biomed. Mater. Res. 66A, 605–614 (2003)
Isenberg, B.C., DiMilla, P.A., Walker, M., Kim, S., Wong, J.Y.: Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys. J. 97, 1313–1322 (2009)
Hadjipanayi, E., Mudera, V., Brown, R.A.: Guiding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motil. Cytoskeleton 66, 121–128 (2009)
Li, S., Butler, P., Wang, Y., Hu, Y., Han, D.C., Usami, S., Guan, J.L., Chien, S.: The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc. Natl. Acad. Sci. USA 99, 3546–3551 (2002)
Riehl, B.D., Lee, J.S., Ha, L., Lim, J.Y.: Fluid-flow-induced mesenchymal stem cell migration: Role of focal adhesion kinase and RhoA kinase sensors. J. R. Soc. Interface 12, 20141351 (2015)
Valignat, M.-P., Neg̀re, P., Cadra, S., Lellouch, A.C., Gallet, F., Heńon, S., Theodoly, O.: Lymphocytes can self-steer passively with wind vane uropods. Nat. Comm. 5, 5213 (2014)
DiMilla, P.A., Barbee, K., Lauffenburger, D.A.: Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys. J. 60, 15–37 (1991)
Dickinson, R.B., Tranquillo, R.T.: A stochastic model for adhesion-mediated cell random motility and haptotaxis. J. Math. Biol. 31, 563–600 (1993)
Gracheva, M.E., Othmer, H.G.: A continuum model of motility in ameboid cells. Bul. Math. Biol. 66, 167–193 (2004)
Zaman, M.H., Kamm, R.D., Matsudaira, P., Lauffenburger, D.A.: Computational model for cell migration in three-dimensional matrices. Biophys. J. 89, 1389–1397 (2005)
Liu, W.K., Liu, Y., Farrell, D., Zhang, L., Wang, X.S., Fukui, Y., Patankar, N., Zhang, Y., Bajaj, C., Lee, J., Hong, J., Chen, X., Hsu, H.: Immersed finite element method and its applications to biological systems. Comput. Methods Appl. Mech. Eng. 195, 1722–1749 (2006)
Lin, Y.: A model of cell motility leading to biphasic dependence of transport speed on adhesive strength. J. Mech. Phys. Solids 58, 502–514 (2010)
Dokukina, I.V., Gracheva, M.E.: A model of fibroblast motility on substrates with different rigidities. Biophys. J. 98, 2794–2803 (2010)
Borau, C., Kamm, R.D., García-Aznar, J.M.: Mechano-sensing and cell migration: A 3D model approach. Phys. Biol. 8, 066008 (2011)
Wong, H.C., Tang, W.C.: Finite element analysis of the effects of focal adhesion mechanical properties and substrate stiffness on cell migration. J. Biomech. 44, 1046–1050 (2011)
Pathak, A., Kumar, S.: From molecular signal activation to locomotion: An integrated, multiscale analysis of cell motility on defined matrices. PLoS ONE 6, e18423 (2011)
Sarvestani, A.S.: A model for cell motility on soft bio-adhesive substrates. J. Biomech. 44, 755–758 (2011)
Shao, D., Levine, H., Rappel, W.-J.: Coupling actin flow, adhesion, and morphology in a computational cell motility model. Proc. Natl. Acad. Sci. USA 109, 6851–6856 (2012)
Schlüter, D.K., Ramis-Conde, I., Chaplain, M.A.J.: Computational modeling of single-cell migration: The leading role of extracellular matrix fibers. Biophys. J. 103, 1141–1151 (2012)
Callan-Jones, A.C., Voituriez, R.: Active gel model of amoeboid cell motility. New J. Phys. 15, 025022 (2013)
Zhong, Y., Ji, B.: Impact of cell shape on cell migration behavior on elastic substrate. Biofabrication 5, 015011 (2013)
Vernerey, F.J., Farsad, M.: A mathematical model of the coupled mechanisms of cell adhesion, contraction and spreading. J. Math. Biol. 68, 989–1022 (2014)
Tarama, M., Yamamoto, R.: Mechanics of cell crawling by means of force-free cyclic motion. J. Phys. Soc. Jpn. 87, 044803 (2018)
Cao, Y., Karmakar, R., Ghabache, E., Gutierrez, E., Zhao, Y., Groisman, A., Levine, H., Camleyd, B.A., Rappel, W.-J.: Cell motility dependence on adhesive wetting. Soft Matter 15, 2043 (2019)
Flaherty, B., McGarry, J.P., McHugh, P.E.: Mathematical models of cell motility. Cell Biochem. Biophys. 49, 14–28 (2007)
Holmes, W.R., Edelstein-Keshet, L.: A comparison of computational models for eukaryotic cell shape and motility. PLoS Comput. Biol. 8, e1002793 (2012)
Shi, C., Huang, C.-H., Devreotes, P.N., Iglesias, P.A.: Interaction of motility, directional sensing, and polarity modules recreates the behaviors of chemotaxing cells. PLoS Comput. Biol. 9, e1003122 (2013)
Danuser, G., Allard, J., Mogilner, A.: Mathematical modeling of eukaryotic cell migration: Insights beyond experiments. Annu. Rev. Cell Dev. Biol. 29, 501–28 (2013)
Ziebert, F, Aranson, I.S.: Computational approaches to substrate-based cell motility. Npj Comput. Mater. 2, 16019 (2016)
Lauffenburger, D.A., Horwitz, A.F.: Cell migration: A physically integrated molecular process. Cell 84, 359–69 (1996)
Gardel, M.L., Schneider, I.C., Aratyn-Schaus, Y., Waterman, C.M.: Mechanical Integration of Actin and Adhesion Dynamics in Cell Migration. Annu. Rev. Cell Dev. Biol. 26, 315–33 (2010)
Takagi, H., Sato, M.J., Yanagida, T., Ueda, M.: Functional analysis of spontaneous cell movement under different physiological conditions. PLoS ONE 3, e2648 (2008)
Huda, S., Weigelin, B., Wolf, K., Tretiakov, K.V., Polev, K., Wilk, G., Iwasa, M, Emami, F.S., Narojczyk, J.W., Banaszak, M., Soh, S., Pilans, D., Vahid, A., Makurath, M., Friedl, P., Borisy, G.G., Kandere-Grzybowska, K., Grzybowski, B.A.: Lévy-like movement patterns of metastatic cancercells revealed in microfabricated systems andimplicated in vivo. Nat. Comm. 9, 4539 (2018)
Stroka, K.M., Gu, Z., Sun, S.X., Konstantopoulos, K.: Bioengineering paradigms for cell migration in confined microenvironment. Curr. Opin. Cell. Biol. 30, 41–50 (2014)
Pathak, A., Kumar, S.: Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. USA 109, 10334–10339 (2012)
Diz-Munõz, A., Fletcher, D.A., Weiner, O.D.: Use the force: Membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 23, 47–53 (2013)
Houk, A.R., Jilkine, A., Mejean, C.O., Boltyanskiy, R., Dufresne, E.R., Angenent, S.B., Altschuler, S.J., Wu, L.F., Weiner, O.D.: Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148, 175–188 (2012)
Ji, L., Lim, M., Danuser, G.: Fluctuations of intracellular forces during cell protrusion. Nat. Cell Biol. 10, 1393–1400 (2008)
Keren, K.: Cell motility: The integrating role of the plasma membrane. Eur. Biophys. J. 40, 1013–1027 (2011)
Dembo, M., Wang, Y.L.: Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 76, 2307–2316 (1999)
Goehring, N.W., Grill, S.W.: Cell polarity: Mechanochemical patterning. Trend Cell Biol. 23, 72–80 (2012)
Jilkine, A., Edelstein-Keshet, L.: A comparison of mathematical models for polarization of single eukaryotic cells in response to guided cues. PLoS Comput. Biol. 7, e1001121 (2011)
Plotnikov, S.V., Pasapera, A.M., Sabass, B., Waterman, C.M.: Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527 (2012)
Lozanne, A.D., Spudich, J.A.: Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236, 1086–1091 (1987)
Spudich, J.A.: One path to understanding energy transduction in biological systems. Nat. Med. 18, 1478–1482 (2012)
Nagel, O., Guven, C., Theves, M., Driscoll, M., Losert, W., Beta1, C.: Geometry-driven polarity in motile amoeboid cells. PLoS ONE 9, e113382 (2014)
Gov, N.S.: Traction forces during collective cell motion. HFSP J. 3, 223–227 (2009)
Stokes, C.L., Lauffenburger, D.A., Williams, S.K.: Migration of individual microvessel endothelial cells: Stochastic model and parameter measurement. J. Cell Sci. 99, 419–430 (1991)
Liu, Y.J., Berre, M.L., Lautenschlaeger, F., Maiuri, P., Callan-Jones, A., Heuzé, M., Takaki, T., Voituriez, R., Piel, M.: Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015)
Kuntz, R.M., Saltzman, W.M.: Neutrophil motility in extracellular matrix gels: Mesh size and adhesion affect speed of migration. Biophys. J. 72, 1472 (1997)
Wacker, B.K., Alford, S.K., Scott, E.A., Thakur, M.D., Longmore, G.D., Elbert, D.L.: Endothelial cell migration on RGD-peptide-containing PEG hydrogels in the presence of sphingosine 1-phosphate. Biophys. J. 94, 273 (2008)
Wu, P., Hoying, J.B., Williams, S.K., Kozikowski, B.A., Lauffenburger, D.A.: Integrin-binding peptide in solution inhibits or enhances endothelial cell migration, predictably from cell adhesion. Ann. Biochem. Eng. 22, 144 (1994)
Bergman, A.J., Zygourakis, K.: Migration of lymphocytes on fibronectin-coated surfaces: temporal evolution of migratory parameters. Biomaterials 20, 2235 (1999)
Harris, A.K., Wild, P., Stopak, D.: Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science 208, 177 (1980)
Maiuri, P., Rupprecht, J.F., Wieser, S., Ruprecht, V., Bénichou, O., Carpi, M., Coppey, M., Beco, S.D., Gov, N., Heisenberg, C.P., Crespo, C.L., Lautenschlaeger, F., Berre, M.L., Lennon-Dumenil, A.M., Raab, M., Thiam, H.R., Piel, M., Sixt, M., Voituriez, R.: Actin flows mediate a universal coupling between cell speed and cell persistence. Cell 161, 374–386 (2015)
Gauthier, N.C., Masters, T.A., Sheetz, M.P.: Mechanical feedback between membrane tension and dynamics. Trend Cell Biol. 22, 527 (2012)
Acknowledgments
The author would like to thank Dr. Shunji Hattori at Nippi Research Institute of Biomatrix for fruitful discussions.
Funding
This work was financially supported by JSPS KAKENHI Grant Number 26800208.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix: A
Theoretical studies referenced in the main text are those focusing on the influence of adhesivity and/or substrate stiffness on the morphology or migration behavior. To compare those studies with the present study, they are briefly reviewed in the below particularly from the viewpoint of model’s characteristics (dimension of the space, continuous or discrete, the number of parameters etc.), calculation procedure (numerical or analytical etc.), and the scope of the study (what is explored among the cell size, speed, persistence and random motility coefficient etc.).
Compared with those studies, the characteristics of the present study can be summarized as follows: The present study aims to investigate, with a single model, as many as three cellular responses, namely the cell length, persistence length, and random motility coefficient, as well as durotaxis and the response to shear stress. In the model, the cell migrates in a 1D space and is represented by its boundaries stochastically changing their positions. The number of parameters, five (Fave, r, K, L0, σ), is the least compared with those studies. Analytical calculation is fully performed to obtain the results represented by some formulae. Thanks to the formulae, we explicitly find the influence of the adhesivity (Fave) and the cellular properties (r, K, L0, σ) on the cellular responses (\(\bar {L}\), ξ, μ) occurring in migration, which lead to the understanding of the mechanism underlying those responses.
The following provides the brief review.
The study [36] is a pioneering work that explores the effect of the adhesivity. The cell is described by coupled springs and dashpots moving in a 1D space. The model includes 13 parameters. The result is numerically obtained. The cell speed is explored.
In [37], the cell motion is described by three points moving in a 2D space. The dynamics is given by stochastic differential equations (SDEs). The model includes 16 parameters. The result is numerically obtained. The scope is the most similar to the present study. The cell speed, persistence and random motility coefficient are explored.
In [38], the cell is described by a continuous finite object in a 1D space. The dynamics is given by partial differential equations (PDEs). The model includes 11 parameters. The result is numerically obtained. The cell size and speed are explored.
In [39], the cell is described by a point moving in a 3D space. Its motion is provided by ordinary differential equations (ODEs). The model includes 9 parameters. The result is numerically obtained. The cell speed is explored.
In [40], the cell is described by a continuous finite object in a 3D space. The dynamics is given by PDEs. Since the PDEs are fully solved with the finite element method, any quantities would be obtained although none of them is explicitly shown. The numbers of parameters is not clearly written in the paper but should be numerous due to the model’s characteristics.
In [41], the cell is represented by the vertical cross section of the cell. The dynamics is given by PDEs. The model includes 10 parameters. While the calculation is proceeded analytically in part, the final results are obtained numerically by solving a system of algebraic equations. The cell speed is explored.
In [42], the cell is described by 95 nodes connected by springs and dashpots placed in a 2D space. Its motion is given by ODEs. The model includes 11 parameters. The results are numerically obtained. The cell speed and cell size are explored in addition to durotaxis.
In [43], the cell is described by an elastic element moving in a 3D space. Its motion is given by ODEs. The model includes 20 parameters. The results are numerically obtained. The effects of substrate stiffness on the traction force and cell speed are explored.
In [44], the cell and substrate are described by continuous substance in a 2D space. The finite element method is used to obtain the result. The model includes 11 parameters. The effects of substrate stiffness on the traction force and cell speed are explored.
In [45], the cell is described by a point in a 1D space. The intracellular processes are provided in detail to determine the motion. The dynamics is given by ODEs. The model includes 25 parameters. The results are numerically obtained. The cell speed is explored.
In [46], the cell is described by a continuous viscoelastic substance in a 1D space. The dynamics is given by PDEs. The model includes 8 parameters. The results are numerically obtained. The effects of the substrate stiffness on the traction force and cell speed are explored.
In [47], the cell is described by a phase field in a 2D space. The dynamics is given by PDEs. The model includes 24 parameters. The results are numerically obtained. The cell morphology is explored.
In [48], the cell is described by a point moving on a 2D space where matrix fibers are distributed. The dynamics is given by ODEs. The model includes 7 parameters. The results are numerically obtained. The effects of the substrate stiffness on the cell speed and persistence are explored as well as durotaxis.
In [49], the cell is described by a continuous active gel in a 1D space. The dynamics is given by PDEs. The model includes 7 parameters. While analytical calculation is performed to understand whether spontaneous movement will occur or not, the cell speed is obtained numerically.
In [50], the boundary of the cell is considered in a 2D space. Time evolution of the cell’s position is not calculated. Instead, the relations between the substrate stiffness, the cell shape and the anisotropy of the adhesive force are explored for the fixed cell. The model includes 14 parameters. The results are numerically obtained.
In [51], the cell is described by a continuous substance in a 2D space. The dynamics is given by PDEs. The model includes 23 parameters. The effect of the substrate stiffness on the cell spreading is explored.
In [52], the cell is described by two points representing the left edge and right edge in a 1D space, which is similar to the present model. The two points are connected with a spring, a dashpot and an active actuator. Periodic motion induced by the periodic attachment and detachment of the adhesion are assumed. The model includes 8 parameters. The result is numerically obtained. The influence of the phase differences of the periodic behavior on the cell velocity is explored.
In [53], 2D cross-section of the cell is considered and represented by a phase field. The dynamics is given by PDEs. The model includes 12 parameters. Results are obtained numerically except for some specific cell forms. The cell speed is explored.
Appendix: B
In the model, for simplicity, the cell is assumed to always switch the migration direction when adhesive force ff is less than membrane tension T, i.e., ff < T. Here, we consider a case where the switching does not always occur. In this case, we should analyze a random walk in which three actions are possible at each time step: 1) the cell keeps the migration direction and moves forward by unit length with probability p, 2) the cell is stationary, i.e., neither moves forward nor switches the migration direction with probability q, and 3) the cell switches the migration direction with probability r. Then the probability of migration forward without switching the migration direction is given by \(p+qp+q^{2}p+\cdots =\frac {p}{1-q}\). Inversely, the probability of switching the migration direction without migrating forward is given by \(r+qr+q^{2}r+\cdots =\frac {r}{1-q}\). Since p + q + r = 1 is satisfied, the ratio of the probability of migrating forward to the probability of switching the migration direction is equal to the ratio that does not involve the probability of the stationary state. Thus, the persistence length calculated in the main text does not change if we assume that switching of the migration direction does not always occur under the condition ff < T.
Appendix: C
Figure 8 shows representative cell trajectories obtained by numerical simulations for three values of mean adhesive force Fm. The cell tends to migrate persistently when it adheres strongly. This figure also shows the time evolution of cell length L(t). After a short period, the cell length reaches an equilibrium state in all cases.
Typical time evolutions of the position of the cell’s left edge (red) and right edge (blue) and the cell length (yellow) at three adhesivities: Fm = 200, 300, and 400 Pa. Stronger adhesion induces higher persistence and larger cell spreading. Other parameter values are r = 1.0 Pa/μ m, K = 20 Pa/μ m, L0 = 10 μ m, and σ = 10 Pa
Appendix: D
The dependence of the cell length L on the adhesive force Fm at different membrane elasticity K and natural cell length L0. Points and lines are respectively obtained from the numerical simulation and the analysis. Error bars representing the standard deviation are short and invisible. Parameter values are r = 1.0 Pa/μ m, K = 20 Pa/μ m, L0 = 10 μ m, and σ = 10 Pa when fixed. The dependences on r and σ are not shown because no dependence is observed as inferred from the analytical results
The dependence of the characteristic persistence length (CPL) ξ on the adhesive force Fm at different polarizability r, membrane elasticity K, natural cell length L0, and adhesivity fluctuation σ. Points and lines are respectively obtained from the numerical simulation and the analysis. Error bars represent the standard deviations. Parameter values are r = 1.0 Pa/μ m, K = 20 Pa/μ m, L0 = 10 μ m, and σ = 10 Pa when fixed
The analytical results derived in the main text and simulation results are compared under a specific combination of parameter values. Figure 9 shows the cell length L. The analytical results well describe the numerical results. Figure 10 shows the characteristic persistence length (CPL) ξ. Analytical results well describe numerical results. Comparison of the random motility coefficients (RMC) μ is not shown since it is proportional to CPL. We can notice a tendency that CPLs obtained numerically are a little smaller than the analytical one. The reason is, since the number of the samples of the simulation is finite, the probability distributions of the persistence length have to be truncated, and therefore the CPL estimated by simulation is smaller than that calculated from the formula obtained by the analysis.
Appendix: E
Figure 11 shows the probability distribution of persistence length Δx, the distance by which the cell moves between direction switches, constructed from the trajectories obtained in numerical simulations. Typical results for three values of adhesive force Fm are shown. All of them are described well by exponential functions, \(\exp (-{\Delta } x/\xi )\), where ξ a parameter referred to as characteristic persistence length.
Probability distributions of persistence length Δx obtained by numerical simulations at three adhesivities: Fm = 200, 300, and 400 Pa. Other parameter values are r = 1.0 Pa/μ m, K = 20 Pa/μ m, L0 = 10 μ m, and σ = 10 Pa. All of them are well described by exponential functions, exp(-Δx/ξ), whose characteristic persistence lengths (CPL) ξ are 3.83, 5.98, 9.84 μ m
Appendix: F
Let us consider a random walk in which the probability of keeping the migration direction is p and the probability of switching the the migration direction is 1 − p. Let N be the number of steps immediately after which the probability of keeping the same migration direction becomes 1/2. Then N satisfies pN = 1/2, which reads \(N=\frac {\log (1/2)}{\log p}\). This means that the probability of keeping the migration direction for sequential N times is 1/2. As a result of this keeping the direction of N times, the distance by which the walker moves is Nξ where ξ denotes the step size of this random walk. Thus, as far as, we focus on the long-time behavior, this asymmetric random walk is equivalent to the symmetric random walk whose unit length \(\tilde {\xi }\) is given by \(\tilde {\xi }=N\xi =\frac {\log (1/2)}{\log p} \xi \).
Appendix: G
Although the random motility coefficient shows a biphasic or monotonically decreasing dependence on increasing adhesive force in the main text, it also manifests an increasing dependence even when we slightly change the relation between adhesive force and cell speed. For example, when we use \(S[\mu \text {m/min}]=20\exp (-F_{m}[\text {Pa}]/200)\) given in Fig. 12 instead of S[μ m/min] = − 0.025Fm[Pa] + 12.5 used in the main text, a monotonically increasing dependence is observed when r is large, K is small, and σ is small (Fig. 13).
The dependence of random motility coefficient on mean adhesive force Fm at different values of polarizability r, membrane elasticity K, natural cell length L0, and adhesion fluctuation σ when an exponential function is used to describe the cell speed. Parameter values are r = 1.0 Pa/μ m, K = 20 Pa/μ m, L0 = 10 μ m, and σ = 10 Pa when fixed
Rights and permissions
About this article
Cite this article
Iwasa, M. A mechanical toy model linking cell-substrate adhesion to multiple cellular migratory responses. J Biol Phys 45, 401–421 (2019). https://doi.org/10.1007/s10867-019-09536-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-019-09536-2