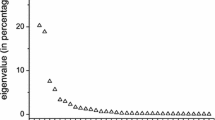
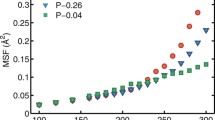
Abstract
Owing to its small size and very fast folding rate, the Trp-cage miniprotein has become a benchmark system to study protein folding. Two folding pathways were found to be characteristic of this protein: pathway I, in which the hydrophobic collapse precedes the formation of α-helix, and pathway II, in which the events occur in the reverse order. At the same time, the relative contribution of these pathways at different temperatures as well as the nature of transition from one pathway to the other remain unclear. To gain insight into this issue, we employ a recently proposed hydrodynamic description of protein folding, in which the process of folding is considered as a motion of a “folding fluid” (Chekmarev et al., Phys. Rev. Lett. 100(1), 018107 2008). Using molecular dynamics simulations, we determine the field of probability fluxes of transitions in a space of collective variables and divide it into stream tubes. Each tube contains a definite fraction of the total folding flow and can be associated with a certain pathway. Specifically, three temperatures were considered, T = 285K, T = 315K, and T = 325K. We have found that as the temperature increases, the contribution of pathway I, which is approximately 90% of the total folding flow at T = 285K, decreases to approximately 10% at T = 325K, i.e., pathway II becomes dominant. At T = 315K, both pathways contribute approximately equally. All these temperatures are found below the calculated melting point, which suggests that the Trp-cage folding mechanism is determined by kinetic factors rather than thermodynamics.








Similar content being viewed by others
References
Neidigh, J.W., Fesinmeyer, R.M., Andersen, N.H.: Designing a 20-residue protein. Nat. Struct. Mol. Biol. 9(6), 425–430 (2002)
Qiu, L., Pabit, S.A., Roitberg, A.E., Hagen, S.J.: Smaller and faster: The 20-residue Trp-cage protein folds in 4 μ s. J. Am. Chem. Soc. 124(44), 12952–12953 (2002)
Ahmed, Z., Beta, I.A., Mikhonin, A.V., Asher, S.A.: UV-resonance Raman thermal unfolding study of Trp-cage shows that it is not a simple two-state miniprotein. J. Am. Chem. Soc. 127(31), 10943–10950 (2005)
Neuweiler, H., Doose, S., Sauer, M.: A microscopic view of miniprotein folding: Enhanced folding efficiency through formation of an intermediate. Proc. Natl. Acad. Sci. USA 102(46), 16650–16655 (2005)
Mok, K.H., Kuhn, L.T., Goez, M., Day, I.J., Lin, J.C., Andersen, N.H., Hore, P.: A pre-existing hydrophobic collapse in the unfolded state of an ultrafast folding protein. Nature 447(7140), 106–109 (2007)
Streicher, W.W., Makhatadze, G.I.: Unfolding thermodynamics of Trp-cage, a 20-residue miniprotein, studied by differential scanning calorimetry and circular dichroism spectroscopy. Biochemistry 46(10), 2876–2880 (2007)
Culik, R.M., Serrano, A.L., Bunagan, M.R., Gai, F.: Achieving secondary structural resolution in kinetic measurements of protein folding: A case study of the folding mechanism of Trp-cage. Angew. Chem. Int. Ed. 123(46), 11076–11079 (2011)
Rovó, P., Farkas, V., Hegyi, O., Szolomájer-Csikós, O., Tóth, G.K., Perczel, A.: Cooperativity network of Trp-cage miniproteins: Probing salt-bridges. J. Pept. Sci. 17(9), 610–619 (2011)
Halabis, A., Zmudzinska, W., Liwo, A., Oldziej, S.: Conformational dynamics of the Trp-cage miniprotein at its folding temperature. J. Phys. Chem. B 116 (23), 6898–6907 (2012)
Lai, Z., Preketes, N.K., Mukamel, S., Wang, J.: Monitoring the folding of Trp-cage peptide by two-dimensional infrared (2dir) spectroscopy. J. Phys. Chem. B 117(16), 4661–4669 (2013)
Meuzelaar, H., Marino, K.A., Huerta-Viga, A., Panman, M.R., Smeenk, L.E., Kettelarij, A.J., van Maarseveen, J.H., Timmerman, P., Bolhuis, P.G., Woutersen, S.: Folding dynamics of the Trp-cage miniprotein: Evidence for a native-like intermediate from combined time-resolved vibrational spectroscopy and molecular dynamics simulations. J. Phys. Chem. B 117(39), 11490–11501 (2013)
Rovó, P., Stráner, P., Láng, A., Bartha, I., Huszár, K., Nyitray, L., Perczel, A.: Structural insights into the Trp-cage folding intermediate formation. Chem. Eur. J. 19(8), 2628–2640 (2013)
Byrne, A., Williams, D.V., Barua, B., Hagen, S.J., Kier, B.L., Andersen, N.H.: Folding dynamics and pathways of the Trp-cage miniproteins. Biochemistry 53 (38), 6011–6021 (2014)
Abaskharon, R.M., Culik, R.M., Woolley, G.A., Gai, F.: Tuning the attempt frequency of protein folding dynamics via transition-state rigidification: Application to Trp-cage. J. Phys. Chem. Lett. 6(3), 521–526 (2015)
Snow, C.D., Zagrovic, B., Pande, V.S.: The Trp-cage: Folding kinetics and unfolded state topology via molecular dynamics simulations. J. Am. Chem. Soc. 124 (49), 14548–14549 (2002)
Pitera, J.W., Swope, W.: Understanding folding and design: Replica-exchange simulations of “Trp-cage” miniproteins. Proc. Natl. Acad. Sci. USA 100, 7587–7592 (2003)
Zhou, R.: Trp-cage: Folding free energy landscape in explicit water. Proc. Natl. Acad. Sci. USA 100(23), 13280–13285 (2003)
Chowdhury, S., Lee, M.C., Duan, Y.: Characterizing the rate-limiting step of Trp-cage folding by all-atom molecular dynamics simulations. J. Phys. Chem. B 108 (36), 13855–13865 (2004)
Linhananta, A., Boer, J., MacKay, I.: The equilibrium properties and folding kinetics of an all-atom go model of the Trp-cage. J. Chem. Phys. 122(11), 114901 (2005)
Juraszek, J., Bolhuis, P.: Sampling the multiple folding mechanisms of Trp-cage in explicit solvent. Proc. Natl. Acad. Sci. USA 103(43), 15859–15864 (2006)
Paschek, D., Hempel, S., García, A.E.: Computing the stability diagram of the Trp-cage miniprotein. Proc. Natl. Acad. Sci. USA 105(46), 17754–17759 (2008)
Marinelli, F., Pietrucci, F., Laio, A., Piana, S.: A kinetic model of Trp-cage folding from multiple biased molecular dynamics simulations. PLoS Comput. Biol. 5 (8), 1000452 (2009)
Day, R., Paschek, D., Garcia, A.E.: Microsecond simulations of the folding/unfolding thermodynamics of the Trp-cage miniprotein. Proteins: Struct. Funct. Bioinf. 78(8), 1889–1899 (2010)
Lindorff-Larsen, K., Piana, S., Dror, R.O., Shaw, D.E.: How fast-folding proteins fold. Science 334(6055), 517–520 (2011)
Zheng, W., Gallicchio, E., Deng, N., Andrec, M., Levy, R.M.: Kinetic network study of the diversity and temperature dependence of Trp-cage folding pathways: Combining transition path theory with stochastic simulations. J. Phys. Chem. B 115 (6), 1512–1523 (2011)
Han, W., Schulten, K.: Further optimization of a hybrid united-atom and coarse-grained force field for folding simulations: Improved backbone hydration and interactions between charged side chains. J. Chem. Theory Comput. 8, 4413–4424 (2012)
Marino, K.A., Bolhuis, P.G.: Confinement-induced states in the folding landscape of the Trp-cage miniprotein. J. Phys. Chem. B 116, 11872–11880 (2012)
Shao, Q., Shi, J., Zhu, W.: Enhanced sampling molecular dynamics simulation captures experimentally suggested intermediate and unfolded states in the folding pathway of Trp-cage miniprotein. J. Chem. Phys. 137(12), 125103 (2012)
Deng, N., Dai, W., Levy, R.M.: How kinetics within the unfolded state affects protein folding: An analysis based on Markov state models and an ultra-long MD trajectory. J. Phys. Chem. B 117(42), 12787–12799 (2013)
Han, W., Schulten, K.: Characterization of folding mechanisms of Trp-cage and WW-domain by network analysis of simulations with a hybrid-resolution model. J. Phys. Chem. B 117(42), 13367–13377 (2013)
Juraszek, J., Saladino, G., Van Erp, T., Gervasio, F.: Efficient numerical reconstruction of protein folding kinetics with partial path sampling and pathlike variables. Phys. Rev. Lett. 110(10), 108106 (2013)
Marinelli, F.: Following easy slope paths on a free energy landscape: The case study of the Trp-cage folding mechanism. Biophys. J. 105(5), 1236–1247 (2013)
Du, W., Bolhuis, P.G.: Sampling the equilibrium kinetic network of Trp-cage in explicit solvent. J. Chem. Phys. 140(19), 195102 (2014)
Kannan, S., Zacharias, M.: Role of tryptophan side chain dynamics on the Trp-cage mini-protein folding studied by molecular dynamics simulations. PloS ONE 9 (2), 88383 (2014)
Mou, L., Jia, X., Gao, Y., Li, Y., Zhang, J.Z., Mei, Y.: Folding simulation of Trp-cage utilizing a new AMBER compatible force field with coupled main chain torsions. J. Theor. Comput. Chem. 13(4), 1450026 (2014)
Orsi, M., Ding, W., Palaiokostas, M.: Direct mixing of atomistic solutes and coarse-grained water. J. Chem. Theory Comput. 10, 4684–4693 (2014)
Kim, S.B., Dsilva, C.J., Kevrekidis, I.G., Debenedetti, P.G.: Systematic characterization of protein folding pathways using diffusion maps: Application to Trp-cage miniprotein. J. Chem. Phys. 142(8), 085101 (2015)
Palese, L.L.: Correlation analysis of Trp-cage dynamics in folded and unfolded states. J. Phys. Chem. B 119, 15568–15573 (2015)
Zhou, C.Y., Jiang, F., Wu, Y.D.: Folding thermodynamics and mechanism of five Trp-cage variants from replica-exchange MD simulations with RSFF2 force field. J. Chem. Theory Comput. 11, 5473–5480 (2015)
Andryushchenko, V.A., Chekmarev, S.F.: A hydrodynamic view of the first-passage folding of Trp-cage miniprotein. Eur. Biophys. J. 45, 229–243 (2016)
Pandini, A., Fornili, A.: Using local states to drive the sampling of global conformations in proteins. J. Chem. Theory Comput. 12, 1368–1379 (2016)
Chekmarev, S.F., Palyanov, A.Y., Karplus, M.: Hydrodynamic description of protein folding. Phys. Rev. Lett. 100(1), 018107 (2008)
Landau, L., Lifshitz, E.: Fluid Mechanics. Pergamon, New York (1987)
Kalgin, I.V., Chekmarev, S.F., Karplus, M.: First passage analysis of the folding of a β-sheet miniprotein: Is it more realistic than the standard equilibrium approach. J. Phys. Chem. B 118(16), 4287–4299 (2014)
Brooks, B.R., Brooks, C.L., MacKerell, A.D., Nilsson, L., Petrella, R.J., Roux, B., Won, Y., Archontis, G., Bartels, C., Boresch, S., Caflisch, A., Caves, L., Cui, Q., Dinner, A.R., Feig, M, Fischer, S., Gao, J., Hodoscek, M., Im, W., Kuczera, K., Lazaridis, T., Ma, J., Ovchinnikov, V., Paci, E., Pastor, R.W., Post, C.B., Pu, J.Z., Schaefer, M., Tidor, B., Venable, R.M., Woodcock, H.L., Wu, X., Yang, W., York, D.M., Karplus, M.: CHARMM: The biomolecular simulation program. J. Comput. Chem. 30(10), 1545–1614 (2009)
Neria, E., Fischer, S., Karplus, M.: Simulation of activation free energies in molecular systems. J. Chem. Phys. 105(5), 1902–1921 (1996)
Ferrara, P., Apostolakis, J., Caflisch, A.: Evaluation of a fast implicit solvent model for molecular dynamics simulations. Proteins Struct. Funct. Bioinf. 46(1), 24–33 (2002)
Ryckaert, J.P., Ciccotti, G., Berendsen, H.J.: Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977)
Privalov, P.L.: Stability of proteins. Small Globular Proteins. Adv. Prot. Chem. 33, 167–241 (1979)
Taverna, D.M., Goldstein, R.A.: Why are proteins marginally stable? Proteins Struct. Funct. Genet. 46, 105–109 (2002)
DuBay, K., Bowman, G.R., Geissler, P.L.: Fluctuations within folded proteins: Implications for thermodynamic and allosteric regulation. Acc. Chem. Res. 48, 1098–1105 (2015)
Tang, Q.Y., Zhang, Y.Y, Wang, J., Wang, W., Chialvo, D.R.: Critical fluctuations in the native state of proteins. Phys. Rev. Lett. 118, 088102 (2017)
Zhou, R.: Free energy landscape of protein folding in water: Explicit vs. implicit solvent. Proteins Struct. Funct. Bioinf. 53(2), 148–161 (2003)
Ferrara, P., Apostolakis, J., Caflisch, A.: Thermodynamics and kinetics of folding of two model peptides investigated by molecular dynamics simulations. J. Phys. Chem. B 104(20), 5000–5010 (2000)
Eaton, W.A., Muñoz, V., Hagen, S.J., Jas, G.S., Lapidus, L.J., Henry, E.R., Hofrichter, J.: Fast kinetics and mechanisms in protein folding. Annu. Rev. Biophys. Biomol. Struct. 29(1), 327–359 (2000)
Jolliffe, I.: Principal Component Analysis. Springer Verlag, New York (2002)
Fraley, C., Raftery, A.E.: Model-based clustering, discriminant analysis, and density estimation. J. Am. Stat. Assoc. 97(458), 611–631 (2002)
Chekmarev, S.F.: Equilibration of protein states: A time dependent free-energy disconnectivity graph. J. Phys. Chem. B 119(26), 8340–8348 (2015)
Andersen, C.A., Palmer, A.G., Brunak, S., Rost, B.: Continuum secondary structure captures protein flexibility. Structure 10, 175–184 (2002)
Seeber, M., Cecchini, M., Rao, F., Settanni, G., Caflisch, A.: WORDOM: A program for efficient analysis of molecular dynamics simulations. Bioinformatics 23 (19), 2625–2627 (2007)
Hanwell, M.D., Curtis, D.E., Lonie, D.C., Vandermeersch, T., Zurek, E., Hutchison, G.R.: Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 4, 1–17 (2012)
Kalgin, I.V., Karplus, M., Chekmarev, S.F.: Folding of a SH3 domain: Standard and “hydrodynamic” analyses. J. Phys. Chem. B 113(38), 12759–12772 (2009)
Kalgin, I.V., Caflisch, A., Chekmarev, S.F., Karplus, M.: New insights into the folding of a beta-sheet miniprotein in a reduced space of collective hydrogen bond variables: Application to a hydrodynamic analysis of the folding flow. J. Phys. Chem. B 117, 6092–6105 (2013)
Kalgin, I.V., Chekmarev, S.F.: Turbulent phenomena in protein folding. Phys. Rev. E 83(1), 011920 (2011)
Landau, L., Lifshitz, E.: Statistical Physics Part 1. Pergamon, New York (1980)
Chekmarev, S.F.: Protein folding: Complex potential for the driving force in a two-dimensional space of collective variables. J. Chem. Phys. 139(14), 145103 (2013)
Baldin, R.L.: Temperature dependence of the hydrophobic interaction in protein folding. Proc. Natl. Acad. Sci. USA 83, 8069–8072 (1986)
Sadqi, M., Lapidus, L.J., Victor Muñoz, V.: How fast is protein hydrophobic collapse. Proc. Natl. Acad. Sci. USA 100, 12117–12122 (2003)
Chan, H.S., Bromberg, S., Dill, K.A.: Models of cooperativity in protein folding. Phil. Trans. R. Soc. Lond. B 348, 61–70 (1995)
Chan, H.S., Zhang, Z., Wallin, S., Liu, Z.: Cooperativity, local-nonlocal coupling, and nonnative interactions: Principles of protein folding from coarse-grained models. Annu. Rev. Phys. Chem. 62, 301–326 (2011)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work was performed under a grant from the Russian Science Foundation (No. 14-14-00325). The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andryushchenko, V.A., Chekmarev, S.F. Temperature evolution of Trp-cage folding pathways: An analysis by dividing the probability flux field into stream tubes. J Biol Phys 43, 565–583 (2017). https://doi.org/10.1007/s10867-017-9470-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-017-9470-7