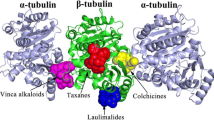
Abstract
Microtubules are formed from the molecules of tubulin, whose dynamics is important for many functions in a cell, the most dramatic of which is mitosis. Taxol is known to interact within a specific site on tubulin and also believed to block cell-cycle progression during mitosis by binding to and stabilizing microtubules. Along with the tremendous potential that taxol has shown as an anticancer drug, clinical problems exist with solubility, toxicity, and development of drug resistance. The crystal structure of taxane diterpenoids, namely, 10, 13-deacetyl-abeo-baccatin-IV (I), 5-acetyl-2-deacetoxydecinnamoyl-taxinine-0.29hydrate (II), 7, 9-dideacetyltaxayuntin (III), and Taxawallin-K (IV), are very similar to the taxol molecule. Considerable attention has been given to such molecules whose archetype is taxol but do not posses long aliphatic chains, to be developed as a substitute for taxol with fewer side effects. In the present work, the molecular docking of these taxane diterpenoids has been carried out with the tubulin alpha-beta dimer (1TUB) and refined microtubule structure (1JFF) using Glide-XP, in order to assess the potential of tubulin binding of these cytotoxic agents. Results show that all the ligands dock into the classical taxol binding site of tubulin. Taxol shows the best binding capabilities. On the basis of docking energy and interactions, apart from taxol, molecule II has a better tendency of binding with 1TUB while molecule I shows better binding capability with bovine tubulin 1JFF. To validate the binding capabilities, molecular dynamics (MD) simulations of the best docked complexes of ligands with 1JFF have been carried out for 15.0 ns using DESMOND. Average RMSD variations and time line study of interactions and contacts indicate that these complexes remain stable during the course of the dynamics. However, taxol and molecule II prevail over other taxoids.






Similar content being viewed by others
References
Pellegrini, F., Bubman, D.R.: Tubulin function, action of antitubulin drugs, and new drug development. Cancer Invest 23, 264–273 (2005)
Kathiravan, G., Sri Raman, V.: In vitro TAXOL production, by Pestalotiopsis breviseta — a first report. Fitoterapia 81, 557–564 (2010)
Schiff, P.B., Fant, J., Horwitz, S.B.: Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–669 (1979)
Giannakakou, P., Gussio, R., Nogales, E., Downing, K.H., Zaharevitz, D., Bollbuck, B., Poyi, G., Sackett, D., Nicolaou, K.C., Fojo, T.: A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. PNAS 97, 2904–2909 (1999)
Wang, H.K., Ohtsu, H., Itokawa, H.: Chemistry of taxol and related taxoids. In: Taxus: the genus Taxus, Ed. Itokawa, H. and Lee, K.H. Taylor & Francis, London. p225 (2003).
Chattopadhyay, S.K., Sharon, A., Yadava, U., Srivastava, S., Mehta, V.K., Maulik, P.R.: A taxane diterpenoid from the needles of Taxus wallichiana. Acta Cryst. E57, o1158–o1160 (2001)
Chattopadhyay, S.K., Sharon, A., Yadava, U., Srivastava, S., Mehta, V.K., Maulik, P.R.: A taxane diterpenoid from the heartwood of Taxus wallichiana. Acta Cryst. E58, o154–o155 (2002)
Yadava, U., Gupta, H., Roychoudhury, M.: A comparison of crystallographic and DFT optimized geometries on two taxane diterpenoids and docking studies with phospholipase A2. Med. Chem. Res. 21, 2162–2168 (2012)
Khan, I., Nisar, M., Ahmad, M., Shah, H., Iqbal, Z., Saeed, M., Muhammad, S., Halimi, A., Kaleem, W.A., Qayum, M., Amana, A., Abdullah, S.M.: Molecular simulations of Taxawallin I inside classical taxol binding site of β-tubulin. Fitoterapia 82, 276–281 (2011)
Nogales, E., Wolf, S.G., Downing, K.H.: Structure of the αβ tubulin dimer by electron crystallography. Nature 391, 199–203 (1998)
Lowe, J., Li, H., Downing, K.H., Nogales, E.: Refined structure of αβ-tubulin at 3.5 Å resolution. J. Mol. Biol 313, 1045–1057 (2001)
Glide: version 5.8, Schrödinger, LLC, New York (2012).
Halgren, T.A., Murphy, R.B., Friesner, R.A., Beard, H.S., Frye, L.L., Pollard, W.T., Banks, J.L.: Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 47, 1750–1759 (2004).
Friesner, R.A., Murphy, R.B., Repasky, M.P., Frye, L.L., Greenwood, J.R., Halgren, T.A., Sanschagrin, P.C., Mainz, D.T.: Extra-Precision Glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 49, 6177–6196 (2006)
Yadava, U., Singh, M., Roychoudhury, M.: Pyrazolo [3,4-d]pyrimidines as inhibitor of anti-coagulation and inflammation activities of phospholipase A2: insight from molecular docking studies. J. Biol. Phys. 39, 419–438 (2013)
Banks, J.L., Beard, H.S., Cao, Y., Cho, A.E., Damm, W., Farid, R., Felts, A.K., Halgren, T.A., Mainz, D.T., Maple, J.R., Murphy, R., Philipp, D.M., Repasky, M.P., Zhang, L.Y., Berne, B.J., Friesner, R.A., Gallicchio, E.: Levy. R.M.: Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comp. Chem. 26, 1752–1780 (2005)
Jorgenson, W.L., Maxwell, D.S., Tirado-Rives, J.: Development and testing of the OPLS all atom force field on conformational energetic and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996)
Sherman, W., Day, T., Jacobson, M.P., Friesner, R.A., Farid, R.: Novel procedure for modelling ligand/receptor induced fit effects. J. Med. Chem. 49, 534–553 (2006)
Li, Y.C., Rissanen, S., Stepniewski, M., Cramariuc, O., Róg, T., Mirza, S., Xhaard, H., Wytrwal, M., Kepczynski, M., Bunker, A.: Study of interaction between PEG carrier and three relevant drug molecules: piroxicam, paclitaxel, and hematoporphyrin. J. Phys. Chem. B 116, 7334–7341 (2012)
Bissantz, C., Folkers, G., Rognan, D.: Protein-based virtual screening of chemical database. 1. Evaluation of different docking/scoring combinations. J. Med. Chem. 43, 4759–4767 (2000).
DESMOND Molecular Dynamics System, version 3.1, D. E. Shaw Research, New York, NY (2012).
Bowers, K.J., Chow, E., Xu, H., Dror, R.O., Eastwood, M.P., Gregersen, B.A., Klepeis, J.L., Kolossvary, I., Moraes, M.A., Sacerdoti, F.D., Salmon, J.K., Shan, Y. and Shaw, D.E.: Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. Proc. ACM/IEEE Conf. Supercomp.(SC06), Tampa, Florida (2006).
Verlet, L.: Computer “experiments” on classical fluids I. Thermodynamical properties of Lennard-Jones molecules. Phys. Rev. 159, 98–103 (1967).
QikProp: version 3.5 Schrödinger, LLC, New York (2012)
Lipinski, C.A.: Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 44, 235–249 (2000)
Jorgensen, W.L., Duffy, E.M.: Prediction of drug solubility from structure. Adv. Drug. Deliv. Rev. 54, 355–366 (2002)
Cramer, G.M., Ford, R.A., Hall, R.L.: Estimation of toxic hazard - a decision tree approach. J. Cosmet. Toxicol. 16, 255–276 (1978)
Srivastava, H.K., Chourasia, M., Kumar, D., Sastry, G.N.: Comparison of computational methods to model DNA minor groove binders. J. Chem. Inf. Model. 51, 558–571 (2011)
Acknowledgments
U. Yadava is thankful to the Department of Science and Technology, New Delhi, for the financial support through its Fast Track Young Scientist Scheme and University Grants Commission, New Delhi for the award of Raman Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2127 kb)
Rights and permissions
About this article
Cite this article
Yadava, U., Gupta, H. & Roychoudhury, M. Stabilization of Microtubules by Taxane Diterpenoids: Insight from Docking and MD simulations. J Biol Phys 41, 117–133 (2015). https://doi.org/10.1007/s10867-014-9369-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-014-9369-5