Abstract
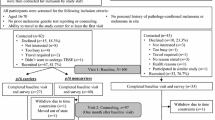
To determine whether receiving melanoma genetic test results undermines perceived control over melanoma prevention, control-related beliefs were examined among 60 adults from melanoma-prone families receiving CDKN2A/p16 test results (27 unaffected noncarriers, 15 unaffected carriers, 18 affected carriers; response rate at 2 years = 64.9 % of eligible respondents). Multilevel modeling of perceived control ratings over a 2-year period revealed significant variation in individual trajectories: most participants showed increases (45 %) or no change (38.3 %), while 16.7 % showed decreases. At the group level, noncarriers reported sustained increases through the 2-year follow-up (ps < .05); unaffected carriers reported significant short-term increases (ps < .05); and affected carriers reported no change. Participants in all groups continued to rate photoprotection as highly effective in reducing melanoma risk and reported decreased beliefs that carrying the p16 mutation would inevitably lead to the development of melanoma. Qualitative responses immediately following counseling and test reporting corroborated these findings, as 93 % indicated it was possible to either prevent (64.9 %) or decrease the likelihood (28.1 %) of future melanomas. Thus, genetic test reporting does not generally undermine perceived control over melanoma prevention, though variability in response to positive results warrants future study.




Similar content being viewed by others
Notes
Because 95 % of participants came from two large extended families or kindreds, we examined whether responses depended on family membership. Because immediate family groups were not mutually exclusive (i.e., a participant could be a brother in one group, but a father in another group), we did not include family unit as a level of nesting. Instead, we accounted for dependencies among members of the same extended family by adding kindred as a level-2 variable in the model testing change in perceived control at 1 month. Of note, the interactions between kindred and participant group were not significant predictors of the intercept, slope for time, or the quadratic effect. Likewise, in a separate model, we found that average changes in perceived control reported by one’s siblings did not predict one’s own changes and did not interact with participant group. As these analyses did not suggest that outcomes depended on family unit, we did not retain either kindred or sibling group as factors in the model.
We considered using latent change score models to evaluate the relationships among difference scores over time (for example, changes in perceived control and changes in protective clothing use). However, these models assume linear relationships and equal time intervals across the assessments, assumptions that are not met by our study design (with assessments at post-counseling, 1, 6, 12, and 24 months) and findings (the curvilinear pattern of increases in perceived control over these time points).
References
Armitage, C. J., & Conner, M. (2001). Efficacy of the theory of planned behaviour: A meta-analytic review. British Journal of Social Psychology, 40, 471–499. doi:10.1348/014466601164939.
Aspinwall, L. G., Brown, T. R., & Tabery, J. (2012). The double-edged sword: Does biomechanism increase or decrease judges’ sentencing of psychopaths? Science, 337, 846–849. doi:10.1126/science.1219569.
Aspinwall, L. G., Leaf, S. L., Dola, E. R., Kohlmann, W., & Leachman, S. A. (2008). CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiology, Biomarkers and Prevention, 17, 1510–1519. doi:10.1158/1055-9965.EPI-08-0010.
Aspinwall, L. G., Leaf, S. L., Kohlmann, W., Dola, E. R., & Leachman, S. A. (2009). Patterns of photoprotection following CDKN2A/p16 genetic test reporting and counseling. Journal of the American Academy of Dermatology, 60, 745–757. doi:10.1016/j.jaad.2008.12.034.
Aspinwall, L. G., Taber, J. M., Kohlmann, W., & Leachman, S. A. (2013a). Psychological aspects of hereditary cancer risk counseling and genetic testing. In B. I. Carr & J. Steel (Eds.), Psychological aspects of cancer: A guide to emotional and psychological consequences of cancer, their causes and their management (pp. 31–64). New York: Springer.
Aspinwall, L. G., Taber, J. M., Kohlmann, W., Leaf, S. L., & Leachman, S. A. (2014a). Perceived risk following melanoma genetic testing: A 2-year prospective study distinguishing subjective estimates from recall. Journal of Genetic Counseling, 23, 421–437. doi:10.1007/s10897-013-9676-1.
Aspinwall, L. G., Taber, J. M., Kohlmann, W., Leaf, S. L., & Leachman, S. A. (2014b). Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genetics in Medicine, 16, 846–853. doi:10.1038/gim.2014.37.
Aspinwall, L. G., Taber, J. M., Leaf, S. L., Kohlmann, W., & Leachman, S. A. (2013b). Genetic testing for hereditary melanoma and pancreatic cancer: A longitudinal study of psychological outcome. Psycho-Oncology, 22, 276–289. doi:10.1002/pon.2080.
Aspinwall, L. G., Taber, J. M., Leaf, S. L., Kohlmann, W., & Leachman, S. A. (2013c). Melanoma genetic counseling and test reporting improve screening adherence among unaffected carriers 2 years later. Cancer Epidemiology, Biomarkers and Prevention, 22, 1687–1697. doi:10.1158/1055-9965.EPI-13-0422.
Bishop, D. T., Demenais, F., Goldstein, A. M., Bergman, W., Bishop, J. N., Bressac-de Paillerets, B., et al. (2002). Geographical variation in the penetrance of CDKN2A mutations for melanoma. Journal of the National Cancer Institute, 94, 894–903. doi:10.1093/jnci/94.12.894.
Cameron, L. D., Marteau, T. M., Brown, P. M., Klein, W. M., & Sherman, K. A. (2012). Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: The role of coherence. Journal of Behavioral Medicine, 35, 286–298. doi:10.1007/s10865-011-9361-5.
Cannon-Albright, L. A., Goldgar, D. E., Meyer, L. J., Lewis, C. M., Anderson, D. E., Fountain, J. W., et al. (1992). Assignment of a locus for familial melanoma, MLM, to chromosome 9p13–p22. Science, 258, 1148–1152. doi:10.1126/science.1439824.
Claassen, L., Henneman, L., De Vet, R., Knol, D., Marteau, T., & Timmermans, D. (2010). Fatalistic responses to different types of genetic risk information: Exploring the role of self-malleability. Psychology & Health, 25, 183–196. doi:10.1080/08870440802460434.
Collins, F. S., Green, E. D., Guttmacher, A. E., & Guyer, M. S. (2003). A vision for the future of genomics research. Nature, 422, 835–847. doi:10.1038/nature01626.
Dar-Nimrod, I., & Heine, S. J. (2011). Genetic essentialism: On the deceptive determinism of DNA. Psychological Bulletin, 137, 800–818. doi:10.1037/a0021860.
Florell, S. R., Boucher, K. M., Garibotti, G., Astle, J., Kerber, R., Mineau, G., et al. (2005). Population-based analysis of prognostic factors and survival in familial melanoma. Journal of Clinical Oncology, 23, 7168–7177. doi:10.1200/JCO.2005.11.999.
Frosch, D. L., Mello, P., & Lerman, C. (2005). Behavioral consequences of testing for obesity risk. Cancer Epidemiology, Biomarkers and Prevention, 14, 1485–1489. doi:10.1158/1055-9965.EPI-04-0913.
Gould, W. A., & Heine, S. J. (2012). Implicit essentialism: Genetic concepts are implicitly associated with fate concepts. PLoS One, 7, e38176. doi:10.1371/journal.pone.0038176.
Graham, J. W. (2009). Missing data analysis: Making it work in the real world. Annual Review of Psychology, 60, 549–576. doi:10.1146/annurev.psych.58.110405.085530.
Kamb, A., Shattuck-Eidens, D., Eeles, R., Liu, Q., Gruis, N. A., Ding, W., et al. (1994). Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nature Genetics, 8, 22–26. doi:10.1038/ng0994-22.
Khoury, M. J., Gwinn, M., Yoon, P. W., Dowling, N., Moore, C. A., & Bradley, L. (2007). The continuum of translation research in genomic medicine: How can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in Medicine, 9, 665–674. doi:10.1097/GIM.0b013e31815699d0.
Lebowitz, M. S., Ahn, W. K., & Nolen-Hoeksema, S. (2013). Fixable or fate? Perceptions of the biology of depression. Journal of Consulting and Clinical Psychology, 81, 518–527. doi:10.1037/a0031730.
Marteau, T. M., French, D. P., Griffin, S. J., Prevost, A. T., Sutton, S., Watkinson, C., et al. (2010). Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Systematic Review. doi:10.1002/14651858.CD007275.pub2.
Marteau, T. M., & Weinman, J. (2006). Self-regulation and the behavioural response to DNA risk information: A theoretical analysis and framework for future research. Social Science and Medicine, 62, 1360–1368. doi:10.1016/j.socscimed.2005.08.005.
McBride, C. M., Bowen, D., Brody, L. C., Condit, C. M., Croyle, R. T., Gwinn, M., et al. (2010). Future health applications of genomics: Priorities for communication, behavioral, and social sciences research. American Journal of Preventive Medicine, 38, 556–565. doi:10.1016/j.amepre.2010.01.027.
Nelkin, D., & Lindee, S. (1995). The DNA mystique. London: WH Freeman & Company.
Parrott, R., Silk, K., Weiner, J., Condit, C., Harris, T., & Bernhardt, J. (2004). Deriving lay models of uncertainty about genes’ role in illness causation to guide communication about human genetics. Journal of Communication, 54, 105–122. doi:10.1111/j.1460-2466.2004.tb02616.x.
Raudenbush, S. W., & Bryk, A. S. (2002). Hierarchical linear models: Applications and data analysis methods (Vol. 1). Thousand Oaks: Sage.
Senior, V., Marteau, T. M., & Weinman, J. (2000). Impact of genetic testing on causal models of heart disease and arthritis: An analogue study. Psychology & Health, 14, 1077–1088. doi:10.1080/08870440008407368.
Ugalde, A., Martin, P., & Rees, G. (2008). Psychological impact of receiving genetic risk information for breast cancer, with and without lifestyle information. Australian Journal of Psychology, 60, 1–9. doi:10.1080/00049530701449497.
Acknowledgments
This work was supported by a Funding Incentive Seed Grant, Office of the Vice President for Research, University of Utah, and a Huntsman Cancer Institute (HCI) CCPS Pilot Project Award to Drs. Aspinwall and Leachman. Additional support was received from the Huntsman Cancer Foundation (HCF), the Tom C. Mathews, Jr. Familial Melanoma Research Clinic endowment, HCI’s Pedigree and Population Resource, the Utah Population Database, and the Utah Cancer Registry, which is funded by contract N01-PC-35141 from the National Cancer Institute (NCI) SEER Program with additional support from the Utah State Department of Health and the University of Utah. The authors used core facilities supported by the National Institutes of Health (NIH) through NCI Cancer Center Support Grant 5P30CA420-14 awarded to HCI, the genetic counseling core facility supported by HCF, and National Center for Research Resources Grant 1KL2RR025763-01 awarded to the University of Utah by the NIH Office of the Director. The authors acknowledge NCI R01 CA158322-01 for partial support during article preparation. Content is the authors’ responsibility and does not necessarily represent views of NCI or NIH. The authors gratefully acknowledge study participants; and Marybeth Hart, Erin Dola, Lisa Wadge, Amber Kostial, Emily Bullough, Michelle Welch, Candace Larson, and Taylor Haskell for contributions to the conduct or management of the study.
Conflict of interest
Ms. Stump, Dr. Taber, Dr. Leaf, and Ms. Kohlmann declare no conflict of interest. Dr. Aspinwall’s work is funded by the NIH. Dr. Leachman’s work is funded by the NIH. Dr. Leachman serves on a Medical and Scientific Advisory Board for Myriad Genetics Laboratory, for which she has received an honorarium. She has collaborated with Myriad on a project to validate an assay that is unrelated to the research reported here.
Human and Animal Rights and Informed Consent
All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aspinwall, L.G., Stump, T.K., Taber, J.M. et al. Impact of melanoma genetic test reporting on perceived control over melanoma prevention. J Behav Med 38, 754–765 (2015). https://doi.org/10.1007/s10865-015-9631-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10865-015-9631-8