Abstract
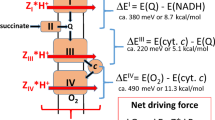
Recently the F0 portion of the bovine mitochondrial F1F0-ATP synthase was shown to contain eight ‘c’ subunits (n = 8). This surprised many in the field, as previously, the only other mitochondrial F0 (for yeast) was shown to have ten ‘c’ subunits. The metabolic implications of ‘c’ subunit copy number explored in this paper lead to several surprising conclusions: (1) Aerobically respiring E. coli (n = 10) and animal mitochondria (n = 8) both have very high F1F0 thermodynamic efficiencies of ≈90 % under typical conditions, whereas efficiency is only ≈65 % for chloroplasts (n = 14). Reasons for this difference, including the importance of transmembrane potential (∆Ψ) as a rotational catalyst, as opposed to an energy source, are discussed. (2) Maximum theoretical P/O ratios in animal mitochondria (n = 8) are calculated to be 2.73 ATP/NADH and 1.64 ATP/FADH2, yielding 34.5 ATP/glucose (assuming NADH import via the malate/aspartate shuttle). The experimentally measured values of 2.44 (±0.15), 1.47 (±0.13), and 31.3 (±1.5), respectively, are only about 10 % lower, suggesting very little energy depletion via transmembrane proton leakage. (3) Finally, the thermodynamic efficiency of oxidative phosphorylation is not lower than that of substrate level phosphorylation, as previously believed. The overall thermodynamic efficiencies of oxidative phosphorylation, glycolysis, and the citric acid cycle are ≈80 % in all three processes.
Similar content being viewed by others
References
Abrahams JP, Leslie AGW, Lutter R, Walker JE (1994) The structure of F1 ATPase from bovine heart mitochondria determined at 2.8 Å resolution. Nature 370:621–628
Akhmedov D, Braun M, Mataki C, Park K-S, Pozzan T, Schoonjans K, Rorsman P, Wollheim CB, Wiederkehr A (2010) Mitochondrial matrix pH controls oxidative phosphorylation and metabolism-secretion coupling in INS-1E clonal cells. FASEB J 24:4613–4626
Arechaga I, Butler PJG, Walker JE (2002) Self-assembly of ATP synthase subunit c rings. FEBS Lett 515:189–193
Ballhausen B, Altendorf K, Deckers-Hebestreit G (2009) Constant c 10 ring stoichiometry in the E. coli ATP synthase analyzed by cross-linking. J Bacteriol 191:2400–2404
Barber J (2009) Photosynthetic energy conversion: natural and artificial. Chem Soc Rev 38:185–196
Bot CT, Prodan C (2010) Quantifying the membrane potential during E. coli growth stages. Biophys Chem 146:133–137
Boyer PD (1993) The binding change mechanism for ATP synthase – some probabilities and possibilities. Biochim Biophys Acta 1140:215–250
Boyer PD (1997) The ATP synthase – a splendid molecular machine. Annu Rev Biochem 66:717–749
Branden M, Sanden T, Brzezinski P, Widengren J (2006) Localized proton microcircuits at the biologicl membrane-water interface. Proc Natl Acad Sci U S A 103:19766–19770
Cherepanov DA, Feniouk BA, Junge W, Mulkidjanian AY (2003) Low dielectric permittivity of water at the membrane interface: effect on the energy coupling mechanism in biological membranes. Biophys J 85:1307–1316
Daum B, Kuhlbrandt W (2011) Electron tomography of plant thylakoid membranes. J Exp Bot 62:2393–2402
Davies KM, Strauss M, Daum B, Kief JH, Osiewacz HD, Rycovska A, Zickermann V, Kühlbrandt W (2011) Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc Natl Acad Sci U S A 108:14121–14126
Davies KM, Anselmi C, Wittig I, Faraldo-Gómez JD, Kühlbrandt W (2012) Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc Natl Acad Sci U S A 109:13602–13607
Duser MG, Zarrabi N, Cipriano DJ, Ernst S, Glick GD, Dunn SD, Borsch M (2009) 36° step size of proton-driven c-ring rotation in F0F1-ATP synthase. EMBO J 28:2689–2696
Ferguson SJ (2000) What dictates the size of a ring? Curr Biol 10:R804–808
Ferguson SJ (2010) ATP synthase: from sequence to ring size to the P/O ratio. Proc Natl Acad Sci U S A 107:16755–16756
Frey TG, Perkins GA, Ellisman MH (2006) Electron tomography of mem-brane-bound cellular organelles. Annu Rev Biophys Biomol Struct 35:199–224
Goodsell DS (1991) Inside a living cell. Trends Biochem Sci 16:203–206
Hauser M, Eichelmann H, Oja V, Heber U, Laisk A (1995) Stimulation by light of rapid pH regulation in the chloroplast stroma in vivo as lndicated by CO, solubilization in leaves. Plant Physiol 108:1059–1066
Heberle J (2000) Proton transfer reactions across bacteriorhodopsin and along the membrane. Biochim Biophys Acta 1458:135–147
Hinkle PC (2005) P/O ratios of mitochondrial oxidative phohsphorylation. Biochim Biophys Acta 1706:1–11
Jang S-y, Kang HT, Hwang ES (2012) Nicotinamide-induced mitophagy event mediated by high NAD/NADH ratio, and SIRT1 protein activation. J Biol Chem 287:19304–19314
Junge W, Sielaff H, Engelbrecht S (2009) Torque generation and elastic power transmission in the rotary F0F1-ATPase. Nature 459:364–370
Kadenbach B (2003) Intrinsic and extrinsic uncoupling gof oxidative phosphorylation. Biochim Biohys Acta 1604:77–94
Kaim G, Dimroth P (1999) ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. EMBO J 18:4118–4127
Khalifat N, Puff N, Bonneau S, Fournier J-B, Angelova MI (2008) Membrane deformation under local pH gradient: mimicking mitochondrial cristae dynamics. Biophys J 95:4924–4933
Kramer DM, Sacksteder CA, Cruz JA (1999) How acidic is the lumen? Photosynth Res 60:151–163
Krebstakies T, Aldag I, Altendorf K, Greie JC, Deckers-Hebestreit G (2008) The stoichiometry of subunit c of E. coli ATP synthase is independent of its rate of synthesis. Biochemistry 47:6907–6916
Krulwich TA, Ito M, Gilmour R, Hicks DB, Guffanti AA (1998) Energetics of alkaliphilic Bacillus species: physiology and molecules. Adv Microb Physiol 40:401–438
Lehninger AL (1965) The mitochondrion. Benjamin, NY, pp 105–120
Lemasters JJ, Grunwald R, Emaus RK (1984) Thermodynamic limits to the ATP/site stoichiometries of oxidative phosphorylation by rat liver mitochondria. J Biol Chem 259:3058–3063
Liu J, Fujisawa M, Hicks DB, Krulwich TA (2009) Characterization of the functionally critical AXAXAXA and PXXEXXP motifs of the ATP synthase c-subunit from an alkaliphilic Bacillus. J Biol Chem 284:8714–8725
Lodish HF (1999) Molecular cell biology. Scientific American Books, NY
Mannella CA (2006) Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 1763:542–548
Mannella CA, Lederer WJ, Jafri MS (2013) The connection between inner membrane topology and mitochondrial function. J Mol Cell Cardiol 62:51–57
Mathews CK, van Holde KE, Appling DR, Anthony-Cahill SJ (2013) Biochemistry, 4th edn. Pearson, Toronto, pp 649–654
Meier T, Morgner N, Matthies D, Pogoryelov D, Keis S, Cook GM, Dimroth P, Bernhard B (2007) A tridecameric c ring of the adenosine triphosphate (ATP) synthase from the thermoalkaliphilic Bacillus sp. strain TA2.A1 facilitates ATP synthesis at low electrochemical proton potential. Mol Microbiol 65:1181–1192
Messer JI, Jackman MR, Willis WT (2004) Pyruvate and citric acid cycle carbon requirements in isolated skeletal muscle mitochondria. Am J Physiol Cell Physiol 286:C565–C572
Metelkin E, Demin O, Kovacs Z, Chinopoulos C (2009) Modeling of ATP-ADP steady-state exchange rate mediated by the adenine nucleotide translocase in isolated mitochondria. FEBS J 276:6942–6955
Meyer Z, Tittingdorf JM, Rexroth S, Schafer E, Schlichting R, Giersch C et al (2004) The stoichiometry of the chloroplast ATP synthase oligomer III in C. reinhardtii is not affected by the metabolic state. Biochim Biophys Acta 1659:92–99
Minakami S, Yoshikawa H (1965) Thermodynamic considerations on erythrocyte glycolysis. Biochem Biophys Res Comm 18:345–349
Mitchell P, Moyle J (1969) Estimation of membrane potential and pH differences across the cristae membrane of rat liver mitochondria. Eur J Biochem 7:471–484
Moran LA, Horton HR, Scrimgeour KG, Perry MD (2012) Principles of biochemistry, 5th edn. Pearson, Boston, pp 433–435
Mukherjee S, Warshel A (2012) Realistic simulations of the coupling between the protonmotive force and the mechanical rotation of the F0-ATPase. Proc Natl Acad Sci U S A 109:14876–14881
Muller DJ, Dencher NA, Meier T, Dimroth P, Suda K, Stahlberg H, Engel A, Seelert H, Matthey U (2001) ATP synthase: constrained stoichiometry of the transmembrane rotor. FEBS Lett 504:219–222
Murakami S, Packer L (1970) Protonation and chloroplast membrane structure. J Cell Biol 47:332–351
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Nelson DL, Cox MM (2013) Lehninger principles of biochemistry, 6th edn. Freeman and Co, NY, pp 755–757
Nicholls DG, Ferguson SJ (2002) Bioenergetics, 3rd edn. Academic Press, Amsterdam, p 83
Noji H, Yasuda R, Yoshida M, Kinosita K (1997) Direct observation of the rotation of F1-ATPase. Nature 386:299–302
Orij R, Postmus J, Beek AT, Brul S, Smits GJ (2009) In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology 155:268–278
Perkins GA, Tjong J, Brown JM, Poquiz PH, Scott RT, Kolson DR, Ellisman MH, Spirou GA (2010) The micro-architecture of mitochondria at active zones: electron tomography reveals novel anchoring scaffolds and cristae structured for high-rate metabolism. J Neurosci 30:1015–1026
Pogoryelov D, Reichen C, Klyszejko AL, Brunisholz R, Muller DJ, Dimroth P, Meier T (2007) The oligomeric state of c rings from cyanobacterial F-ATP synthases varies from 13 to 15. J Bacteriol 189:5895–5902
Pogoryelov D, Yildiz O, Faraldo-Gomez JD, Meier T (2009) High-resolution structure of the rotor ring of a proton-dependent ATP synthase. Nat Struct Mol Biol 16:1068–1073
Pogoryelov D, Klyszejko AL, Krasnoselska GO, Heller E-M, Leone V, Langer JD, Vonck J, Muller DJ, Faraldo-Gomez JD, Meier T (2012) Engineering rotor ring stoichiometries in the ATP synthase Proc. Proc Natl Acad Sci U S A 109:E1599–E1608
Polgar O, Robey RW, Morisaki K, Dean M, Michejda C, Sauna ZE, Ambudkar SV, Tarasova N, Bates SE (2004) Mutational analysis of ABCG2: role of the GXXXG motif. Biochemistry 43:9448–9456
Porcelli AM, Ghelli A, Zann C, Pinton P, Rizzuto R, Rugolo M (2005) pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun 326:799–804
Preiss L, Klyszejko AL, Hicks DB, Liu J, Fackelmayer OJ, Yildiz O, Krulwich TA, Meier T (2013) The c-ring stoichiometry of ATP synthase is adapted to cell physiological requirements of alkaliphilic Bacillus pseudofirmus OF4. Proc Natl Acad Sci U S A 110:7874–7879
Riondet C, Cachon R, Wache Y, Alcarza G, Divies C (1997) Measurement of the intracellular pH in Escherichia coli with the internally conjugated fluorescent probe 5- (and 6-)carboxyfluorescein succinimidyl ester. Biotechnol Tech 11:735–738
Robinson SP, Giersch C (1987) Inorganic phosphate concentration in the stroma of isolated chloroplasts and its influence on photosynthesis. Aust J Plant Physiol 14:451–462
Rolfe DFS, Brand MD (1997) The physiological significance of proton leak in animal cells and tissues. Biosci Rep 17:9–16
Rottenberg H (1979) Non-equilibrium thermodynamics of energy conversion in bioenergetics. Biochim Biphys Acta 549:225–253
Russ WP, Engelman DM (2000) The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 296:911–919
Schemidt RA, Qu J, Williams JR, Brusilow WSA (1998) Effects of carbon source on expression of F0 genes and on the stoichiometry of the c subunit in the F1F0 ATPase of E. coli. J Bacteriol 180:3205–3208
Senes A, Gerstein M, Engelman DM (2000) Structure of MsbA from Vibrio cholera: a multidrug resistance ABC transporter homolog in a closed conformation. J Mol Biol 296:921–936
Silverstein T (2005) The mitochondrial phosphate-to-oxygen ratio is not an integer. Biochem Mol Biol Educ 33:416–417
Silverstein TP et al (1993) Transmembrane measurements across bioenergetic membranes. Biochim Biophys Acta 1183:1–3
Soboll S, Stucki J (1985) Regulation of the degree of coupling of oxidative phosphorylation in intact rat liver. Biochim Biophys Acta 807:245–254
Staehlin LA, Arntzen CJ (1983) Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol 97:1327–1337
Steigmuller S, Turina P, Graber P (2008) The thermodynamic H+/ATP ratios of the H+-ATP-synthases from chloroplasts and E. coli. Proc Natl Acad Sci U S A 105:3745–3750
Stitt M, McC. Lilley R, Heldt HW (1982) Adenine nucleotide levels in the cytosol, chloroploasts, and mitochondria of wheat leaf protoplasts. Plant Physiol 70:971–977
Stock D, Leslie AGW, Walker JE (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286:1700–1705
Strauss M, Hofhaus G, Schroder RR, Kuhlbrandt W (2008) Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J 27:1154–1160
Symersky J, Pagadala V, Osowski D, Krah A, Meier T, Faraldo-Gomez JD, Mueller DM (2012) Structure of the c10 ring of the yeast mitochondrial ATP synthase in the open conformation. Nat Struct Mol Biol 19:485–491
Toyabe S, Watanabe-Nakayama T, Okamoto T, Kudo S, Muneyuki E (2011) Thermodynamic efficiency and mechanochemical coupling of F1-ATPase. Proc Natl Acad Sci U S A 108:17951–17956
Tran QH, Unden G (1998) Changes in the proton potential and the cellular energetics of Escherichia coli during growth by aerobic and anaerobic respiration or by fermentation. Eur J Biochem 251:538–543
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Voet D, Voet JG (2004) Biochemistry, 3rd edn. J. Wiley and Sons, NY, p 807
von Ballmoos C, Cook GM, Dimroth P (2008) Unique rotary ATP synthase and its biological diversity. Annu Rev Biophys 37:43–64
von Ballmoos C, Wiedenmann A, Dimroth P (2009) Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem 78:649–672
Watt IN, Montgomery MG, Runswick MJ, Leslie AGW, Walker JE (2010) Bioenergetic cost of making an adeonsine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci U S A 107:16823–16827
Wikstrom M, Hummer G (2012) Stoichiometry of proton translocation by respiratory complex I and its mechanistic implications. Proc Natl Acad Sci U S A 109:4431–4436
Zala D, Hinckelmann M-V, Yu H, da Cunha MML, Liot G, Cordelieres FP, Marco S, Saudou F (2013) Vesicular glycolysis provides on-board energy for fast axonal transport. Cell 152:479–491
Zilberstein D, Agmon V, Schuldiner S, Padan E (1984) Escherichia coli intracellular pH, membrane potential, and cell growth. J Bacteriol 158:246–252
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Error propagation in thermodynamic calculations in Table 1
Taking the high-potential E. coli as an example, the experimental measurements are: Δψ = − 220(±22)mV; ΔpH = + 0.9(±0.09); [Pi] = 1(±0.1)mM; [ATP]/[ADP] = 10(±0.14).
H+ flow
∆μH+ is calculated from Eq. 2: F(−0.22 V) − 2.3RT(0.9) = − 6.3 kcal/mol H+.
The uncertainty in ∆μH+ is then: SQRT[(F(0.022 V))2 + (2.3RT(0.09))2] = ± 0.52kcal/mol.
∆GH+ is calculated from Eq. 3: − 6.3 kcal/mol H+ × 10/3 = − 21 kcal.
The uncertainty in ∆GH+ is then: 0.52 kcal/mol × 10/3 = ± 1.7 kcal
ATP synthesis
Q’ for ATP synthesis is calculated from Eq. 5: 10/0.001 = 10,000 M−1.
The relative uncertainty in Q’ is then: SQRT[(0.14/10)2 + (0.1/1)2] = ± 0.101, and the absolute uncertainty in Q ’ = 10, 000 × 0.101 = ± 1010 M− 1.
∆GATP synth. is calculated from Eq. 6: 7.6 + RTln(10, 000) = + 13.056 kcal/mol.
The uncertainty in ∆GATP synth. is then: RT(1010/10000) = ± 0.06 kcal/mol.
Net reaction: thermodynamic efficiency
The thermodynamic efficiency is calculated from Eq. 7: 13.056/21 = 62.2 %.
The relative uncertainty in the thermodynamic efficiency is then:
SQRT[(0.06/13.056)2 + (0.52/21)2] = 0.0830, and the absolute uncertainty in the efficiency = 0.0830 × 62.2 % = 5.2 %
Appendix 2: Calculations of Q’ and ∆G
For the reactions presented in Tables 4 and 5, the balanced equations are as follows:
-
1.
glucose combustion: C6H12O6 + 6 O2 ➔ 6 CO2 + 6 H2O
The relevant local concentrations are cytoplasmic for glucose, but mitochondrial matrix for O2 and CO2: Glucose is catabolized by glycolytic enzymes in the cytoplasm, whereas the mitochondrial matrix is the site of O2 consumption and CO2 release.
-
2.
pyruvate combustion: C3H4O3 + 5/2 O2 ➔ 3 CO2 + 2 H2O
-
3.
ET chains, FADH2 combustion: FADH2 + ½ O2 ➔ FAD + H2O
-
4.
ET chains, NADH combustion: NADH + H+ + ½ O2 ➔ NAD+ + H2O
-
5.
ATP synthesis: ATP4− + H2O ➔ ADP3− + HOPO3 2− + H+
Q’ for ATP synthesis is calculated with cytoplasmic concentrations for glycolytic ATP; for citric acid cycle and oxidative phosphorylation ATP, mitochondrial matrix concentrations are used. These concentrations are listed in Table 3. For glucose combustion, typical concentrations (from Table 3) are:
-
cytoplasmic glucose: 5.0 mM; matrix O2: 25 μM; matrix CO2: 1.1 mM,
-
so for glucose combustion, \( Q\hbox{'}=\frac{{\left[0.0011\right]}^6}{\left[0.0050\right]{\left[25\left({10}^{-6}\right)\right]}^6}=1.77\left(1{0}^{-18}\right)/1.22\left(1{0}^{-30}\right)=1.45\left(1{0}^{12}\right) \)
-
At 37 °C, \( \begin{array}{l}\mathrm{RTlnQ}'=0.001987\;\mathrm{kcal}/\mathrm{mol}\cdotp \mathrm{K}\times 310.15\;\mathrm{K}\times \ln \left(1.45\left(1{0}^{12}\right)\right)=\hfill \\ {}\kern.1em 0.616\times 28.0=17.(3)\;\mathrm{kcal}/\mathrm{mol}\hfill \end{array} \),
-
so ΔG = ΔG° ’ + RTlnQ ’ = − 687 + 17. (3) = − 670 kcal/mol
Metabolite concentrations vary quite widely with the metabolic state of the cell, and also with tissue type. A reasonable range of variation would be five-fold above and below the typical steady state values cited in Table 3. From this, a range of Q’ can be calculated. For glucose combustion, the high-end Q’ would be
Conversely, the low-end Q’ would be
Thus, in different tissues and different physiological conditions, the value of ∆G for glucose combustion can be estimated to range ± 13 kcal/mol around the ‘normal’ value of −670 kcal/mol. Similar ranges of Q’ and ∆G can be calculated for most of the reactions in Table 4. For the ATP hydrolysis reaction, the phosphate concentration and [ATP]/[ADP] ratio vary somewhat less than other metabolites (Stitt et al. 1982; Metelkin et al. 2009; Minakami and Yoshikawa 1965; Soboll and Stucki 1985; Lemasters et al. 1984. Values five-fold above and below the steady state [ATP]/[ADP] ratio, and four times above and below the steady state phosphate concentration were used to calculate, for ATP hydrolysis, Q’ = [Pi] ÷ ([ATP]/[ADP]).
[Pi] ÷ ([ATP]/[ADP]) | Q’ | ∆G (kcal/mol) | ||
Cytoplasm: | Q’ (avg.): | \( \frac{0.0010}{10} \) | 1.0 × 10−4 | −13.3 |
Q’ (low): | \( \frac{0.0010/4}{10\times 5} \) | 5.0 × 10−6 | −15.1 | |
Q’ (high): | \( \frac{0.0010\times 4}{10/5} \) | 2.0 × 10−3 | −11.4 | |
Matrix: | Q’ (avg.): | \( \frac{0.010}{3} \) | 3.33 × 10−3 | −11.1 |
Q’ (low): | \( \frac{0.010/4}{3\times 5} \) | 1.67 × 10−4 | −13.0 | |
Q’ (high): | \( \frac{0.010\times 4}{3/5} \) | 6.67 × 10−2 | −9.3 |
Rights and permissions
About this article
Cite this article
Silverstein, T.P. An exploration of how the thermodynamic efficiency of bioenergetic membrane systems varies with c-subunit stoichiometry of F1F0 ATP synthases. J Bioenerg Biomembr 46, 229–241 (2014). https://doi.org/10.1007/s10863-014-9547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-014-9547-y