Abstract
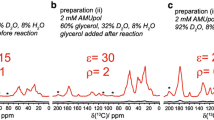
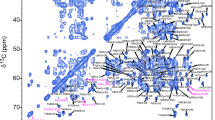
We here investigate the interactions between the DnaB helicase and the C-terminal domain of the corresponding DnaG primase of Helicobacter pylori using solid-state NMR. The difficult crystallization of this 387 kDa complex, where the two proteins interact in a six to three ratio, is circumvented by simple co-sedimentation of the two proteins directly into the MAS-NMR rotor. While the amount of information that can be extracted from such a large protein is still limited, we can assign a number of amino-acid residues experiencing significant chemical-shift perturbations upon helicase-primase complex formation. The location of these residues is used as a guide to model the interaction interface between the two proteins in the complex. Chemical-shift perturbations also reveal changes at the interaction interfaces of the hexameric HpDnaB assembly on HpDnaG binding. A structural model of the complex that explains the experimental findings is obtained.



Similar content being viewed by others
References
Abdul Rehman SA, Verma V, Mazumder M et al (2013) Crystal structure and mode of helicase binding of the C-terminal domain of primase from Helicobacter pylori. J Bacteriol 195:2826–2838. doi:10.1128/JB.00091-13
Bailey S, Eliason WK, Steitz TA (2007) Structure of hexameric DnaB helicase and its complex with a domain of DnaG primase. Science 318:459–463. doi:10.1126/science.1147353
Bazin A, Cherrier MV, Gutsche I et al (2015) Structure and primase-mediated activation of a bacterial dodecameric replicative helicase. Nucleic Acids Res. doi:10.1093/nar/gkv792
Bertini I, Luchinat C, Parigi G et al (2011) Solid-state NMR of proteins sedimented by ultracentrifugation. Proc Natl Acad Sci 108:10396–10399. doi:10.1073/pnas.1103854108
Böckmann A, Gardiennet C, Verel R et al (2009) Characterization of different water pools in solid-state NMR protein samples. J Biomol NMR 45:319–327. doi:10.1007/s10858-009-9374-3
Corn JE, Berger JM (2006) Regulation of bacterial priming and daughter strand synthesis through helicase-primase interactions. Nucleic Acids Res 34:4082–4088. doi:10.1093/nar/gkl363
Gardiennet C, Schütz AK, Hunkeler A et al (2012) A sedimented sample of a 59 kDa dodecameric helicase yields high-resolution solid-state NMR spectra. Angew Chem Int Ed Engl 51:7855–7858. doi:10.1002/anie.201200779
Kashav T, Nitharwal R, Abdulrehman SA et al (2009) Three-dimensional structure of N-terminal domain of DnaB helicase and helicase-primase interactions in Helicobacter pylori. PLoS ONE 4:e7515. doi:10.1371/journal.pone.0007515
Mainz A, Jehle S, van Rossum BJ et al (2009) large protein complexes with extreme rotational correlation times investigated in solution by magic-angle-spinning NMR spectroscopy. J Am Chem Soc 131:15968–15969. doi:10.1021/ja904733v
Oakley AJ, Loscha KV, Schaeffer PM et al (2005) Crystal and solution structures of the helicase-binding domain of Escherichia coli primase. J Biol Chem 280:11495–11504. doi:10.1074/jbc.M412645200
Parsonnet J (1995) Bacterial infection as a cause of cancer. Environ Health Perspect 103(Suppl 8):263–268
Scholz I, Huber M, Manolikas T et al (2008) MIRROR recoupling and its application to spin diffusion under fast magic-angle spinning. Chem Phys Lett 460:278–283. doi:10.1016/j.cplett.2008.05.058
Stelter M, Gutsche I, Kapp U et al (2012) Architecture of a dodecameric bacterial replicative helicase. Structure 20:554–564. doi:10.1016/j.str.2012.01.020
Stevens TJ, Fogh RH, Boucher W et al (2011) A software framework for analysing solid-state MAS NMR data. J Biomol NMR 51:437–447. doi:10.1007/s10858-011-9569-2
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234
Su X-C, Schaeffer PM, Loscha KV et al (2006) Monomeric solution structure of the helicase-binding domain of Escherichia coli DnaG primase. FEBS J 273:4997–5009. doi:10.1111/j.1742-4658.2006.05495.x
Syson K, Thirlway J, Hounslow AM et al (2005) Solution structure of the helicase-interaction domain of the primase DnaG: a model for helicase activation. Struct Fold Des 13:609–616. doi:10.1016/j.str.2005.01.022
Takegoshi K, Nakamura S, Terao T (2001) dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett 344:631–637. doi:10.1016/S0009-2614(01)00791-6
Wiegand T, Gardiennet C, Ravotti F et al (2015) Solid-state NMR sequential assignments of the N-terminal domain of HpDnaB helicase. Biomol NMR Assign. doi:10.1007/s12104-015-9629-8
Williamson MP (2013) Using chemical shift perturbation to characterise ligand binding. Prog Nucl Magn Reson Spectrosc 73:1–16. doi:10.1016/j.pnmrs.2013.02.001
Acknowledgments
This work was supported by the French Agence Nationale de la Recherche (ANR-11-BSV8-021-01, ANR-12-BS08- 0013-01, ANR-14-CE09-0024B), the ETH Zurich, the Swiss National Science Foundation (Grants 200020_159707 and 200020_146757). AB and LT are supported by the CIBLE program 2011 from the Région Rhône-Alpes. We thank Professor Gourinath for providing us the coordinates of the HpDnaG-CTD model. We are grateful for support from TGIR-RMN-THC FR3050.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Carole Gardiennet and Thomas Wiegand have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gardiennet, C., Wiegand, T., Bazin, A. et al. Solid-state NMR chemical-shift perturbations indicate domain reorientation of the DnaG primase in the primosome of Helicobacter pylori . J Biomol NMR 64, 189–195 (2016). https://doi.org/10.1007/s10858-016-0018-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-016-0018-0