Abstract
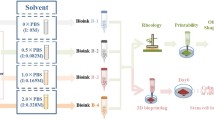
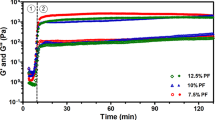
Alginate–gelatin (Alg–Gel) composite hydrogel is extensively used in extrusion-based bioprinting. Although Alg–Gel blends possess excellent biocompatibility and printability, poor mechanical properties have hindered its further clinical applications. In this study, a series of design by incorporating bioactive glass nanoparticles (BG) (particle size of 12 and 25 nm) into Alg–Gel hydrogel have been considered for optimizing the mechanical and biological properties. The composite Alg–Gel–BG bioink was biophysically characterized by mechanical tests and bioprinting practice. Biocompatibility of Alg–Gel–BG bioink was then investigated by bioprinting mouse dermal fibroblasts. Mechanical tests showed enhanced stiffness with increasing concentration of incorporated BG. But the maximum concentration of BG was determined 1.0 wt% before blends became too viscous to print. Meanwhile, the incorporation of BG did not affect the highly porous structure and biodegradation of Alg–Gel hydrogel, while the mechanical strength and printability were enhanced. In addition, the cellular proliferation and adhesion in the bioprinted constructs were significantly enhanced by BG (12 nm), while extension was not affected. Therefore, our strategy of incorporating BG in Alg–Gel composite hydrogel represents an easy-to-use approach to the mechanical reinforcement of cell-laden bioink, thus demonstrating their suitability for future applications in extrusion-based bioprinting.








Similar content being viewed by others
References
Panwar A, Tan LP. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules. 2016;21:685.
Moroni L, Boland T, Burdick JA, Maria CD, Derby B, Forgacs G, et al. Biofabrication: a guide to technology and terminology. Trends Biotechnol.2018;36:384–402.
Axpe E, Oyen ML. Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci. 2016;17:1976.
Pi Q, Maharjan S, Yan X, Liu X, Singh B, van Genderen AM, et al. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv Mater. 2018;30:e1706913.
Freeman FE, Kelly DJ. Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues. Sci Rep. 2017;7:17042.
Faulkner-Jones A, Fyfe C, Cornelissen DJ, Gardner J, King J, Courtney A, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7:044102.
Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials. 2012;33:3279–305.
Nemati S, Rezabakhsh A, Khoshfetrat AB, Nourazarian A, Avci CB, Bagca BG, et al. Alginate-gelatin encapsulation of human endothelial cells promoted angiogenesis in in vivo and in vitro milieu. Biotechnol Bioeng. 2017;114:2920–30.
Lewandowska-Łańcucka J, Mystek K, Mignon A, van Vlierberghe S, Latkiewicz A, Nowakowska M, et al. Alginate- and gelatin-based bioactive photocross-linkable hybrid materials for bone tissue engineering. Carbohydr Polym. 2017;157:1714–22.
Lopes S, Bueno L, Júnior FA, Finkler C. Preparation and characterization of alginate and gelatin microcapsules containing Lactobacillus rhamnosus. An Acad Bras Cienc. 2017;89:1601–13.
Aljohani W, Ullah MW, Zhang X, Yang G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int J Biol Macromol. 2018;107:261–75.
Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3:144–56.
Jiang T, Munguia-Lopez J, Flores-Torres S, Grant J, Vijayakumar S, De Leon-Rodriguez A, et al. Bioprintable alginate/gelatin hydrogel 3D in vitro model systems induce cell spheroid formation. J Vis Exp. 2018;137:e57826.
Müller M, Becher J, Schnabelrauch M, Zenobi-Wong M. Nanostructured pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication. 2015;7:035006.
Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–30.
Wang C, Xie Y, Li A, Shen H, Wu D, Qiu D. Bioactive nanoparticle through postmodification of colloidal silica. ACS Appl Mater Interfaces. 2014;6:4935–9.
Wang C, Shen H, Tian Y, Xie Y, Li A, Ji L, et al. Bioactive nanoparticle-gelatin composite scaffold with mechanical performance comparable to cancellous bones. ACS Appl Mater Interfaces. 2014;6:13061–8.
Zhu F, Wang C, Yang S, Wang Q, Liang F, Liu C, et al. Injectable tissue adhesive composite hydrogel with fibroblasts for treating skin defects. J Mater Chem B. 2017;5:2416–24.
Santos JM, Pereira S, Sequeira D, Messias AL, Martins JB, Cunha H, et al. Biocompatibility of a bioceramic silicone-based sealer in subcutaneous tissue. J Oral Sci. 2019;61:171–7.
Li Z, Huang S, Liu Y, Yao B, Hu T, Shi H, et al. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci Rep. 2018;8:8020
Cheng L, Yao B, Hu T, Cui X, Shu X, Tang S, et al. Properties of an alginate-gelatin-based bioink and its potential impact on cell migration, proliferation, and differentiation. Int J Biol Macromol. 2019;135:1107–13.
Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810.
Wei DX, Dao JW, Chen GQ. A micro-Ark for cells: highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration. Adv Mater. 2018;30:e1802273.
Pati F, Jang J, Ha DH, Kim SW, Rhie JW, Shim JH, et al. Printing three dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Gu Q, Tomaskovic-Crook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, et al. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv Healthc Mater. 2016;5:1429–38.
Hench LL. The story of bioglass. J Mater Sci Mater Med. 2006;17:967–78.
Leite AJ, Sarker B, Zehnder T, Silva R, Mano JF, Boccaccini AR. Bioplotting of a bioactive alginate dialdehyde-gelatin composite hydrogel containing bioactive glass nanoparticles. Biofabrication. 2016;8:035005
Christodoulou I, Buttery LD, Saravanapavan P, Tai G, Hench LL, Polak JM. Dose- and time-dependent effect of bioactive gel-glass ionic-dissolution products on human fetal osteoblast-specific gene expression. J Biomed Mater Res B Appl Biomater. 2005;74:529–37.
McKee CT, Last JA, Russell P, Murphy CJ. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng Part B Rev. 2011;17:155–64.
Acknowledgements
Gratitude to Prof. Dong Qiu (Institute of Chemistry, Chinese Academy of Sciences, China) for providing BG in this research. Gratitude to Liju Xu, Chen Wang, and Qirui Guo (Institute of Chemistry, Chinese Academy of Sciences, China) for their help in mechanical tests and analysis. This study was supported in part by the National Nature Science Foundation of China (81830064, 81721092, and 81701906), the National Key Research and Development Plan (2017YFC1103300), Funds Chinese PLA General Hospital for Military Medical Innovation Research Project (CX19026), the CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-059), the Military Medical Research and Development Projects (AWS17J005, 2019-126), and the Fostering Funds of Chinese PLA General Hospital for National Distinguished Young Scholar Science Fund (2017-JQPY-002).
Author information
Authors and Affiliations
Contributions
LW, ZL, and SH were responsible for the design and primary technical process, conducted the experiments, collected and analyzed data. ZL and LW wrote the paper. JL, YZ, BY, YL, and WS helped perform the main experiments. XF, XW, and SH collectively oversaw the collection of data and data interpretation, and revised the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, L., Li, Z., Li, J. et al. An approach for mechanical property optimization of cell-laden alginate–gelatin composite bioink with bioactive glass nanoparticles. J Mater Sci: Mater Med 31, 103 (2020). https://doi.org/10.1007/s10856-020-06440-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06440-3