Abstract
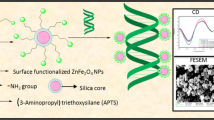
Magnetic nanoparticles (MNPs) especially iron oxide (Fe3O4) NPs have quite extensively been used for in vivo delivery of biomolecules and drugs because of their high bioconjugation efficiency. In this study, Fe3O4 NPs and (3-Aminopropyl) triethoxysilane (APTS) coated Fe3O4 NPs were synthesized and their interaction with Calf thymus (Ct) DNA has been studied in order to understand their usage in biomedical applications. Hydrothermal method was used for the NPs synthesis. Characterization of NPs was done using techniques like UV-Visible spectroscopy, FTIR spectroscopy, FE-SEM, EDAX, Zeta Sizer and powder XRD. Further, interaction studies of NPs with Ct-DNA were investigated using various physicochemical techniques. In UV-Visible studies, hypochromicity with binding constant 3.2 × 105 M−1 was observed. Binding constants calculated using fluorescence studies were found to be k = 3.2 × 104 M−1, 2.9 × 104 M−1 at 293 and 323 K respectively. Results of UV-Visible and fluorescence studies were in correlation with other techniques like UV-TM and CD. All studies suggested alteration in DNA conformation on interaction with surface engineered Fe3O4 NPs, stabilizing DNA-NPs conjugate via partial intercalation and electrostatic interactions. This study may facilitate our understanding regarding the physicochemical properties and DNA-binding ability of APTS-Fe3O4 NPs for their further application in magnetosensitive biosensing and drug delivery.

Iron oxide based magnetic nanoparticles are well known for their excellent bio-conjugation efficiency and therefore APTS-Fe3O4 NPs were synthesized via very simple and benign hydrothermal method. Further, the interaction of APTS-Fe3O4 NPs with calf thymus DNA was studied using various physicochemical techniques to explore their potential in biomedical applications.









Similar content being viewed by others
References
Ali A, Hira Zafar MZ, ul Haq I, Phull AR, Ali JS, Hussain A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl. 2016;9:49–67.
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L. et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemicalcharacterizations, and biological applications. Chem Rev. 2008;108(6):2064–110.
Engelmann U, Buhl EM, Baumann M, Schmitz-Rode T, Slabu I.Agglomeration of magnetic nanoparticles and its effects on magnetic hyperthermia. Curr Dir Biomed Eng. 2017;3(2):457–60.
Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021.
Li K, Nejadnik H, Daldrup-Link HE. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov Today. 2017;1(22):1421–9.
Pershina AG, Sazonov AE, Filimonov VD. Magnetic nanoparticles–DNA interactions: design and applications of nanobiohybrid systems. Russian Chem Rev. 2014;83(4):299.
Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene ther. 2002;9(2):102–9.
Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11(11):2319–31.
Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302.
Verma IM, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–42.
Berry CC, Curtis AS. Functionalization of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2016;36:R198.
Wu W, Wu Z, Yu T, Jiang C, Kim WS. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2015;16:023501.
Tiwari AP, Ghosh SJ, Pawar SH. Biomedical applications based on magnetic nanoparticles: DNA interactions. Anal Methods. 2015;7:10109–20.
Shen M, Cai H, Wang X, Cao X, Li K, Wang SH. et al. Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology. 2012;23(10):105601.
Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano lett. 2013;13(3):1059–64.
Sohrabijam Z, Saeidifar M, Zamanian A. Enhancement of magnetofection efficiency using chitosan coated superparamagnetic iron oxide nanoparticles and calf thymus DNA. Colloids and Surfaces B. Biointerfaces. 2017;152:169–75.
Yadav N, Singh A, Kaushik M. Synthesis and characterization of hydrothermally synthesized superparamagnetic APTS-ZnFe2O4 nanoparticles: DNA binding studies for exploring biomedical applications. Chemical Papers. 2020;74(4):1177–88.
Komal, Sonia, Kukreti S., Kaushik M. Exploring the potential of environment friendly silver nanoparticles for DNA interaction: physicochemical approach. J Photochem Photobiol B Biol. 2019;194:158–65.
Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3:397.
Giri S, Trewyn BG, Stellmaker MP, Lin VS. Stimuli‐responsive controlled‐release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angewandte Chemie. 2005;117(32):5166–72.
Carvalho MD, Henriques F, Ferreira LP, Godinho M, Cruz MM. Iron oxide nanoparticles: the influence of synthesis method and size on composition and magnetic properties. J Solid State Chem. 2013;201:144–52.
Sarkar N, Sharma RS, Kaushik M. Green synthesis and physiochemical characterization of nickel oxide nanoparticles: Interaction studies with Calf thymus DNA. Luminescence. 2020;35(2):178–86.
Souza TGF, Ciminelli VST, Mohallem NDS. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J Phy Conf Ser. 2016;733:012039
Saion E, Gharibshahi E, Naghavi K. Size-controlled and optical properties of monodispersed silver nanoparticles synthesized by the radiolytic reduction method. Int J Mol Sci. 2013;14:7880–96.
Isnaeni, Irmaniar, Herbani Y. Aggregation effect on absorbance spectrum of laser ablated gold nanoparticles. J Phy Conf Ser. 2017;817:012039.
KR SG, Mathew BB, Sudhamani CN, Naik HB. Mechanism of DNA binding and cleavage. J Biomed Biotechnol. 2014;2:1–9.
Sarwar T, Rehman SU, Husain MA, Ishqi HM, Tabish M. Interaction of coumarin with calf thymus DNA: deciphering the mode of binding by in vitro studies. Int J Biol Macromol. 2015;73:9–16.
Qais FA, Abdullah KM, Alam MM, Naseem I, Ahmad I. Interaction of capsaicin with calf thymus DNA: A multi-spectroscopic and molecular modelling study. Int J Biol Macromol. 2017;97:392–402.
Shahabadi N, Hadidi S. Spectroscopic studies on the interaction of calf thymus DNA with the drug levetiracetam. Spectrochim Acta A. 2012;96:278–83.
Sonia, Komal, Kukreti S, Kaushik M. Exploring the DNA damaging potential of chitosan and citrate-reduced gold nanoparticles: physicochemical approach. Int J Biol Macromol. 2018;115:801–10.
Singh A, Neelam, Kaushik M. Physicochemical investigations of zinc oxide nanoparticles synthesized from Azadirachta Indica (Neem) leaf extract and their interaction with Calf-Thymus DNA. Results Phys. 2019;13:102168.
Bhattacharya S, Mandal G, Ganguly T. Detailed spectroscopic investigations to reveal the nature of interaction of anionic porphyrin with calf thymus DNA. J Photoch Photobio B. 2010;101(1):89–96.
Das S, Kumar GS. Molecular aspects on the interaction of phenosafranine to deoxyribonucleic acid: model for intercalative drug-DNA binding. J Mol Struct. 2008;872:56–63.
Roy S, Sadhukhan R, Ghosh U, Das TK. Interaction studies between biosynthesized silver nanoparticle with calf thymus DNA and cytotoxicity of silver nanoparticles. Spectrochim Acta A. 2015;141:176–84.
Chen H, Li S, Yao Y, Zhou L, Zhao J, Gu Y, et al. Design, synthesis, and anti-tumor activities of novel triphenylethylene–coumarin hybrids, and their interactions with Ct-DNA. Bioorg Med Chem Lett. 2013;23(17):4785–9.
Shahabadi N, Fatahi N, Mahdavi M, Nejad ZK, Pourfoulad M. Multispectroscopic studies of the interaction of calf thymus DNA with the anti-viral drug, valacyclovir. Spectrochim Acta A. 2011;83(1):420–4.
Sarwar T, Ishqi HM, Rehman SU, Husain MA, Rahman Y, Tabish M. Caffeic acid binds to the minor groove of calf thymus DNA: A multi-spectroscopic, thermodynamics and molecular modelling study. Int J Biol Macromol. 2017;98:319–28.
Bera R, Sahoo BK, Ghosh KS, Dasgupta S. Studies on the interaction of isoxazol curcumin with calf thymus DNA. Int J Biol Macromol. 2008;42(1):14–21.
Pang JY, Qin Y, Chen WH, Luo GA, Jiang ZH. Synthesis and DNA-binding affinities of monomodified berberines. Bioorg Med Chem. 2005;13(20):5835–40.
Shahabadi N, Fili SM, Kheirdoosh F. Study on the interaction of the drug mesalamine with calf thymus DNA using molecular docking and spectroscopic techniques. J Photoch Photobio B. 2013;128:20–6.
Maddili SK, Katla R, Kannekanti VK, Bejjanki NK, Tuniki B, Zhou CH, et al. Molecular interaction of novel benzothiazolyl triazolium analogues with calf thymus DNA and HSA-their biological investigation as potent antimicrobial agents. Eur J Med Chem. 2018;150:228–47.
Rehman SU, Yaseen Z, Husain MA, Sarwar T, Ishqi HM, Tabish M. Interaction of 6 mercaptopurine with calf thymus DNA–deciphering the binding mode and photoinduced DNA damage. PLoS ONE. 2014;9(4):e93913.
Husain MA, Ishqi HM, Rehman SU, Sarwar T, Afrin S, Rahman Y, et al. Elucidating the interaction of sulindac with calf thymus DNA: biophysical and in silico molecular modelling approach. New J Chem. 2017;41(24):14924–35.
Xu X, Wang D, Sun X, Zeng S, Li L, Sun D. Thermodynamic and spectrographic studies on the interactions of ct-DNA with 5-fluorouracil and tegafur. Thermochimica acta. 2009;493(1–2):30–6.
Sahoo BK, Ghosh KS, Bera R, Dasgupta S. Studies on the interaction of diacetylcurcumin with calf thymus-DNA. Chem Phys. 2008;351(1–3):163–9.
Acknowledgements
Authors sincerely acknowledge Prof. S. Kukreti (Department of Chemistry) for constantly encouraging authors by extending his lab facilities to them. Also, authors are thankful to Prof. H.P. Singh (Director, Cluster Innovation Centre, Delhi University) and Prof. R. Chandra (Head, Department of Chemistry, Delhi University) for their constant support. MK appreciates the financial support from DU-DST Purse grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yadav, N., Singh, A. & Kaushik, M. Hydrothermal synthesis and characterization of magnetic Fe3O4 and APTS coated Fe3O4 nanoparticles: physicochemical investigations of interaction with DNA. J Mater Sci: Mater Med 31, 68 (2020). https://doi.org/10.1007/s10856-020-06405-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06405-6