Abstract
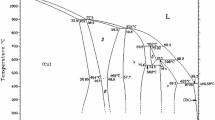
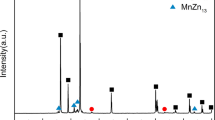
To reduce the long-term side effects of permanent metallic stents, a new generation of cardiovascular stents called “biodegradable stents” is being extensively developed. Zinc has been considered as a promising candidate material for biodegradable cardiovascular stents due to its excellent biocompatibility and appropriate biodegradability. However, weak mechanical properties limit its further clinic application. In this study, hot extruded pure Zn and Zn-0.02 Mg alloy were prepared. Compared with pure Zn, Zn-0.02 Mg alloy showed more homogeneous microstructure, much smaller grain size and higher mechanical strength. Zn-0.02 Mg alloy presented uniform corrosion morphologies during the immersion process, and its corrosion rates was higher than that of pure Zn. Hemocompatibility results showed that the Zn-based alloy had extremely low hemolysis rate (0.74 ± 0.15%) and strong inhibitory effect on blood coagulation, platelet adhesion and aggregation. Zn-0.02 Mg alloy also exhibited excellent cytocompatibility. Its extracts could significantly promote the proliferation of endothelial cells. Moreover, the antibacterial activities of the Zn-based alloy were demonstrated by spread plate assay, live/dead viability assay and bacterial morphology observation. These results indicate that the extruded Zn-0.02 Mg alloy has a potential in biodegradable cardiovascular stents.










Similar content being viewed by others
References
Laslett LJ, Alagona P, Clark BA, Drozda JP, Saldivar F, Wilson SR, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1–49.
Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98.
Hu T, Yang J, Cui K, Rao Q, Yin T, Tan L, et al. Controlled slow-release drug-eluting stents for the prevention of coronary restenosis: recent progress and future prospects. ACS Appl Mater Interfaces. 2015;7:11695–712.
Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–907.
Hu T, Lin S, Du R, Fu M, Rao Q, Yin T, et al. Design, preparation and performance of a novel drug-eluting stent with multiple layer coatings. Biomater Sci. 2017;5:1845–57.
Kereiakes DJ, Cox DA, Hermiller JB, Midei MG, Bachinsky WB, Nukta ED, et al. Usefulness of a cobalt chromium coronary stent alloy. Am J Cardiol. 2003;92:463–6.
Barth KH, Virmani R, Froelich J, Takeda T, Lossef SV, Newsome J, et al. Paired comparison of vascular wall reactions to Palmaz stents, Strecker tantalum stents, and Wallstents in canineiliac and femoral arteries. Circulation. 1996;93:2161–9.
Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115:2426–34.
Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105:2974–80.
Finn AV, Virmani R. The clinical challenge of disappearing stents. Lancet. 2016;387:510–2.
Chung WS, Park CS, Seung KB, Kim PJ, Lee JM, Koo BK, et al. The incidence and clinical impact of stent strut fractures developed after drug-eluting stent implantation. Int J Cardiol. 2008;125:325–31.
Waksman R. Update on bioabsorbable stents: from bench to clinical. J Inter Cardiol. 2006;19:414–21.
Yue Y, Wang L, Yang N, Huang J, Lei L, Ye H, et al. Effectiveness of biodegradable magnesium alloy stents in coronary artery and femoral artery. J Inter Cardiol. 2015;28:358–64.
Bowen PK, Shearier ER, Zhao S, Guillory RJ, Zhao F, Goldman J, et al. Biodegradable metals for cardiovascular stents: from clinical concerns to recent Zn-alloys. Adv Health Mater. 2016;5:1121–40.
Wykrzykowska JJ, Kraak RP, Hofma SH, van der Schaaf RJ, Arkenbout EK, IJsselmuiden AJ, et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. 2017;376:2319–28.
Montone RA, Niccoli G, De Marco F, Minelli S, D’Ascenzo F, Testa L, et al. Temporal trends in adverse events after everolimus-eluting bioresorbable vascular scaffold versus everolimus-eluting metallic stent implantation: a meta-analysis of randomized controlled trials. Circulation. 2017;135:2145–54.
Zheng YF, Gu XN, Witte F. Biodegradable metals. Mater Sci Eng R. 2014;77:1–34.
Gu X, Zheng Y, Cheng Y, Zhong S, Xi T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30:484–98.
Silva CLP, Oliveira AC, Costa CGF, Figueiredo RB, Leite MF, Pereira MM, et al. Effect of severe plastic deformation on the biocompatibility and corrosion rate of pure magnesium. J Mater Sci. 2017;52:5992–6003.
Pierson D, Edick J, Tauscher A, Pokorney E, Bowen P, Gelbaugh J, et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J Biomed Mater Res B Appl Biomater. 2012;100:58–67.
Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411.
Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–7.
Powell SR, Hambidge M, Cousins RJ, Costello RB. The antioxidant properties of zinc. J Nutr. 2000;130:1447s–54s.
Meika F, Samir S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4:676–94.
Meerarani P, Reiterer G, Toborek M, Hennig B. Zinc modulates PPARγ21 signaling and activation of porcine endothelial cells. J Nutr. 2003;133:3058–64.
Hennig B, Toborek M, Mcclain CJ. Antiatherogenic properties of zinc: implications in endothelial cell metabolism. Nutrition. 1996;12:711–7.
Bowen PK, Drelich J, Goldman J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv Mater. 2013;25:2577–82.
Bowen PK, Guillory RJ, Shearier ER, Seitz JM, Drelich J, Bocks M, et al. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater Sci Eng C Mater Biol Appl. 2015;56:467–72.
Drelich AJ, Zhao S, Guillory RJ, Drelich JW, Goldman J. Long-term surveillance of zinc implant in murine artery: surprisingly steady biocorrosion rate. Acta Biomater. 2017;58:539–49.
Yang H, Wang C, Liu C, Chen H, Wu Y, Han J, et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials. 2017;145:92–105.
Gong H, Wang K, Strich R, Zhou JG. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J Biomed Mater Res B Appl Biomater. 2015;103:1632–40.
Wang LQ, Ren YP, Sun SN, Zhao H, Li S, Qin GW. Microstructure, mechanical properties and fracture behavior of as-extruded Zn-Mg binary alloys. Acta Met Sin. 2017;30:931–40.
Li HF, Xie XH, Zheng YF, Cong Y, Zhou FY, Qiu KJ, et al. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci Rep. 2015;5:10719.
ASTM, ASTM-E8/E8m-11: standard test methods for tension testing of metallic materials, annual book of ASTM standards. ASTM, USA; 2011.
ASTM, ASTM-G102-89: standard practice for calculation of corrosion rates and related information from electrochemical measurements. annual book of ASTM standards. ASTM, USA; 2004.
ASTM, ASTM-G31-72: standard practice for laboratory immersion corrosion testing of metals, annual book of ASTM standards. ASTM, USA; 2004.
IOS, ISO 10993-12: biological evaluation of medical devices-part 12: sample preparation and reference materials. IOS, Switzerland; 2012.
Lin S, Wang Q, Yan X, Ran X, Wang L, Zhou JG, et al. Mechanical properties, degradation behaviors and biocompatibility evaluation of a biodegradable Zn-Mg-Cu alloy for cardiovascular implants. Mater Lett. 2019;234:294–7.
ASTM, ASTM-F756-08: Standard Practice for Assessment of Hemolytic Properties of Materials, Annual Book of ASTM Standards. ASTM, USA; 2008.
IOS, ISO 10993-5: biological evaluation of medical devices-part 5: tests for in vitro cytotoxicity. IOS, Switzerland; 2009.
Hermawan H, Dubé D, Mantovani D. Developments in metallic biodegradable stents. Acta Biomater. 2010;6:1693–7.
Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301.
Monchaux E, Vermette P. Effects of surface properties and bioactivation of biomaterials on endothelial cells. Front Biosci. 2010;2:239–55.
Huang N, Yang P, Leng YX, Chen JY, Sun H, Wang J, et al. Hemocompatibility of titanium oxide films. Biomaterials. 2003;24:2177–87.
Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–9.
Ning C, Wang X, Li L, Zhu Y, Li M, Yu P, et al. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: implications for a new antibacterial mechanism. Chem Res Toxicol. 2015;28:1815–22.
Phan TN, Buckner T, Sheng J, Baldeck JD, Marquis RE. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol. 2004;19:31–8.
Scheie AA, Assev S, Rölla G. Combined effect of xylitol, NaF and ZnCl2 on growth and metabolism of Streptococcus sobrinus OMZ 176. APMIS. 1988;96:761.
Acknowledgements
The work was supported by grants from the National Key Technology R & D Program of China (2016YFC1102305), Chongqing Science and Technology Bureau (cstc2019jcyj-zdxm0033), the National Natural Science Foundation of China (11332003). We are also thankful for the support from the Chongqing Engineering Laboratory in Vascular Implants, the Public Experiment Centre of State Bioindustrial Base (Chongqing) and the National “111 plan” (B06023). The authors also acknowledges the kind assistance of Dr Haijun Zhang and MSc Yuxia Yin from National United Engineering Laboratory for Biomedical Material Modification, China in collection and analysis of experimental data as well as discussion on revised manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, S., Ran, X., Yan, X. et al. Systematical evolution on a Zn–Mg alloy potentially developed for biodegradable cardiovascular stents. J Mater Sci: Mater Med 30, 122 (2019). https://doi.org/10.1007/s10856-019-6324-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6324-9