Abstract
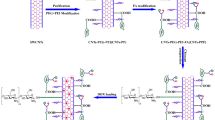
A novel dual-drug delivery system (DDDS) for cancer chemotherapy has been established by employing highly purified and mildly oxidized large-inner-diameter multi-walled carbon nanotubes (LID-MWCNTs) as the vector. The LID-MWCNTs were modified with the antitumor drugs, cisplatin (CDDP) and doxorubicin (DOX). CDDP was encapsulated inside the nanotube vectors by a wet-chemical approach while DOX was attached to the external surfaces through non-covalently interaction. The loading efficiencies of CDDP and DOX were as high as 84.56 and 192.67%, respectively. Notably, after CDDP was encapsulated inside the nanotubes, a three-level blocking strategy, which included polyethylene glycol, folic acid and DOX, was employed to block the CDDP exits at different levels. The pH-sensitive release profile of CDDP was demonstrated using a modified characterization method, as well as that of DOX. Finally, the anticancer activity of the DDDS on MCF-7 cells was tested and a synergistic effect was recorded. This work is part of our LID-MWCNTs based drug delivery system studies, and provides a basis for developing a novel comprehensive antitumor treatment that combines chemotherapy and photothermal therapy.
Graphical Abstract






Similar content being viewed by others
References
Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17(17-18):1044–52. doi:10.1016/j.drudis.2012.05.010
Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomedicine. 2007;3(1):20–31. doi:10.1016/j.nano.2006.11.008
Zhang X, Guo S, Fan R, Yu M, Li F, Zhu C, et al. Dual-functional liposome for tumor targeting and overcoming multidrug resistance in hepatocellular carcinoma cells. Biomaterials. 2012;33(29):7103–14. doi:10.1016/j.biomaterials.2012.06.048
Chun R, Kurzman ID, Couto CG, Klausner J, Henry C, MacEwen EG. Cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma: a pilot study. J Vet Intern Med. 2000;14(5):495–8. doi:10.1111/j.1939-1676.2000.tb02265.x
Guthrie TH Jr., Porubsky ES, Luxenberg MN, Shah KJ, Wurtz KL, Watson PR. Cisplatin-based chemotherapy in advanced basal and squamous cell carcinomas of the skin: results in 28 patients including 13 patients receiving multimodality therapy. J Clin Oncol. 1990;8(2):342–6.
Scambia G, DeVincenzo R, Ranelletti FO, Panici PB, Ferrandina G, Dagostino G, et al. Antiproliferative effect of silybin on gynaecological malignancies: synergism with cisplatin and doxorubicin. Eur J Cancer. 1996;32A(5):877–82. doi:10.1016/0959-8049(96)00011-1
Xu S-P, Sun G-P, Shen Y-X, Wei W, Peng W-R, Wang H. Antiproliferation and apoptosis induction of paeonol in HepG(2) cells. World J Gastroenterol. 2007;13(2):250–6.
Meng L, Zhang X, Lu Q, Fei Z, Dyson PJ. Single walled carbon nanotubes as drug delivery vehicles: targeting doxorubicin to tumors. Biomaterials. 2012;33(6):1689–98. doi:10.1016/j.biomaterials.2011.11.004
Du Z, Zhang S, Zhou C, Liu M, Li G. Dynamic layer-by-layer self-assembly of multi-walled carbon nanotubes on quartz wool for on-line separation of lysozyme in egg white. Talanta. 2012;94(0):104–10. doi:10.1016/j.talanta.2012.03.002
Ren Y, Pastorin G. Incorporation of hexamethylmelamine inside capped carbon nanotubes. Adv Mater.. 2008;20(11):2031–6. doi:10.1002/adma.200702292
Dhar S, Liu Z, Thomale Jr, Dai H, Lippard SJ. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc. 2008;130(34):11467–76. doi:10.1021/ja803036e
Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33(11):3324–33. doi:10.1016/j.biomaterials.2012.01.025
Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56(6):1194
Wike JL. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2(4):343
Du C, Deng D, Shan L, Wan S, Cao J, Tian J, et al. A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials. 2013;34(12):3087–97. doi:10.1016/j.biomaterials.2013.01.041
Yin Q, Shen J, Zhang Z, Yu H, Li Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv Drug Delivery Rev. 2013;65(13-14):1699–715. doi:10.1016/j.addr.2013.04.011
Zhang X, Meng L, Lu Q, Fei Z, Dyson PJ. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 2009;30(30):6041–7. doi:10.1016/j.biomaterials.2009.07.025
Sui L, Yang T, Gao P, Meng A, Wang P, Wu Z, et al. Incorporation of cisplatin into PEG-wrapped ultrapurified large-inner-diameter MWCNTs for enhanced loading efficiency and release profile. Int J Pharm. 2014;471(1–2):157–65. doi:10.1016/j.ijpharm.2014.05.022
Liu J, Zhong L, Zhang J, Luo T, Zhou J, Zhao X, et al. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials. 2016;83:51–65.
Ma G, Zhang C, Zhang L, Sun H, Song C, Wang C, et al. Doxorubicin-loaded micelles based on multiarm star-shaped PLGA–PEG block copolymers: influence of arm numbers on drug delivery. J Mater Sci: Mater Med. 2015;27(1):17 doi:10.1007/s10856-015-5610-4
Chen Q. Functional nanomaterials of NaYF4: Yb/Er- MWCNT: synthesis, characterization and applications in targeted diagnosis and therapy of tumor cell. Scientia Sinica Chimica. 2011;41(5):798 doi:10.1360/032010-860
Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1–8. doi:10.1002/ijc.24336
Tekade RK, Dutta T, Gajbhiye V, Jain NK. Exploring dendrimer towards dual drug delivery: pH responsive simultaneous drug-release kinetics. J Microencapsul. 2009;26(4):287–96. doi:10.1080/02652040802312572
Katragadda U, Teng Q, Rayaprolu BM, Chandran T, Tan C. Multi-drug delivery to tumor cells via micellar nanocarriers. Int J Pharm. 2011;419(1-2):281–6. doi:10.1016/j.ijpharm.2011.07.033
Li J, Yap SQ, Yoong SL, Nayak TR, Chandra GW, Ang WH, et al. Carbon nanotube bottles for incorporation, release and enhanced cytotoxic effect of cisplatin. Carbon. 2012;50(4):1625–34. doi:10.1016/j.carbon.2011.11.043
Tripisciano C, Kraemer K, Taylor A, Borowiak-Palen E. Single-wall carbon nanotubes based anticancer drug delivery system. Chem Phys Lett. 2009;478(4-6):200–5. doi:10.1016/j.cplett.2009.07.071
Wang J. Effects of ultrasonic radiation intensity on the oxidation of single-walled carbon nanotubes in a mixture of sulfuric and nitric acids. Nano. 2013;8(4):46–54.
Guven A, Rusakova IA, Lewis MT, Wilson LJ. Cisplatin@US-tube carbon nanocapsules for enhanced chemotherapeutic delivery. Biomaterials. 2012;33(5):1455–61. doi:10.1016/j.biomaterials.2011.10.060
Li J, Yap SQ, Chin CF, Tian Q, Yoong SL, Pastorin G, et al. Platinum(IV) prodrugs entrapped within multiwalled carbon nanotubes: selective release by chemical reduction and hydrophobicity reversal. Chem Sci. 2012;3(6):2083–7. doi:10.1039/c2sc01086k
Liu Z, Sun X, Nakayama-Ratchford N, Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1(1):50–56. doi:10.1021/nn700040t
Niu L, Meng L, Lu Q. Folate-conjugated PEG on single walled carbon nanotubes for targeting delivery of doxorubicin to cancer cells. Macromol Biosci. 2013;13(6):735–44. doi:10.1002/mabi.201200475
Huang H, Yuan Q, Shah JS, Misra RD. A new family of folate-decorated and carbon nanotube-mediated drug delivery system: synthesis and drug delivery response. Adv Drug Deliv Rev. 2011;63(14-15):1332–9. doi:10.1016/j.addr.2011.04.001
Cheng F-F, Zhang J-J, Xu F, Hu L-H, Abdel-Halim ES, Zhu J-J. pH-Sensitive polydopamine nanocapsules for cell imaging and drug delivery based on folate receptor targeting. J Biomed Nanotechnol. 2013;9(7):1155–63. doi:10.1166/jbn.2013.1611
Yi P, Chen KL. Influence of surface oxidation on the aggregation and deposition kinetics of multiwalled carbon nanotubes in monovalent and divalent electrolytes. Langmuir. 2011;27(7):3588–99. doi:10.1021/la104682b
Saleh NB, Pfefferle LD, Elimelech M. Aggregation kinetics of multiwalled carbon nanotubes in aquatic systems: measurements and environmental implications. Environ Sci Technol. 2008;42(21):7963–9. doi:10.1021/es801251c
Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73(9):2432
Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski V Jr, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52(12):3396
Wang Y, Yang S-T, Wang Y, Liu Y, Wang H. Adsorption and desorption of doxorubicin on oxidized carbon nanotubes. Colloids Surf B. 2012;97:62–69. doi:10.1016/j.colsurfb.2012.04.013
Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–8. doi:10.1038/nnano.2008.111
Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110(2):442–8.
Grubek-Jaworska H, Nejman P, Czumińska K, Przybyłowski T, Huczko A, Lange H, et al. Preliminary results on the pathogenic effects of intratracheal exposure to one-dimensional nanocarbons. Carbon. 2006;44(6):1057–63.
Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, et al. Induction of mesothelioma in p53 mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33:105–16.
Bianco A, Kostarelos K, Prato M. Making carbon nanotubes biocompatible and biodegradable. Chem Commun. 2011;47(37):10182–8.
Lacerda L, Ali-Boucettal H, Herrero MA, Pastorin G, Bianco A, Prato M, et al. Tissue histology and physiology following intravenous administration of different types of functionalized multiwalled carbon nanotubes. Nanomedicine. 2008;3(2):149–61. doi:10.2217/17435889.3.2.149
Fraczek A, Menaszek E, Paluszkiewicz C, Blazewicz M. Comparative in vivo biocompatibility study of single- and multi-wall carbon nanotubes. Acta Biomater. 2008;4(6):1593–602.
Gulati N, Gupta H. Two faces of carbon nanotube: toxicities and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst. 2012;29(1):65–88.
Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68(16):6652–60.
Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4(11):747–51.
Allen BL, Kichambare PD, Gou P, Vlasova II, Kapralov AA, Konduru N, et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8(11):3899–903.
Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5(5):354–9.
Acknowledgements
We would like to thank Pro Yunmao Liao and Dr. Xibo Pei for their constructive discussion. This work was supported by Tianjin Natural Science Foundation (grant no. 16JCZDJC32800) and Sichuan Natural Science Foundation (grant no. 2014SZ0201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Tao Yang and Zhenzhen Wu contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yang, T., Wu, Z., Wang, P. et al. A large-inner-diameter multi-walled carbon nanotube-based dual-drug delivery system with pH-sensitive release properties. J Mater Sci: Mater Med 28, 110 (2017). https://doi.org/10.1007/s10856-017-5920-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5920-9