Abstract
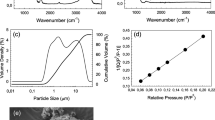
In the present study, novel glasses xSrO–(10−x) MgO–60SiO2–20CaO–10 P2O5 (2 ≤ x ≤ 8, in steps of 2) are synthesized via sol–gel method. The current work focusses on the evaluation of mechanical, physical and biocompatible properties for sol–gel glasses. The pore size and surface area of these glasses were studied using BET analysis. The structural aspect of the glasses/glass ceramics was studied by XRD and Raman spectroscopy. The cytotoxicity assays were conducted for MG63 human osteosarcoma cell line. Furthermore, the as prepared glasses were used for the fabrication of 3-D porous scaffolds via polymer replication method. The loaded green bodies have been sintered at 700, 800 and 900 °C and were kept for 6 h to densify the glass network. The effect of sintering temperature on the structure and properties of as prepared scaffolds were analyzed via scanning electron microscopy (SEM) and porosity calculations.
Graphical Abstract













Similar content being viewed by others
References
Jones JR, Ehrenfried LM, Hench LL. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27:964–73.
Fu Q, Rahaman MN, Bal BS, Brown RF, Day DE. Mechanical and in vitro performance of 13-93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008;4:1854–64.
Bellucci D, Sola A, Cannillo V. A revised replication method for bioceramic scaffolds. Bioceram Devolp App. 2011;1:1–8.
Deliormanli AM, Rahaman MN. Direct-write assembly of silicate and borate bioactive glass scaffolds for bone rep air. J Eur Ceram Soc. 2012;32:3637–46.
Baino F, Brovarone CV. Three-dimensional glass-derived scaffolds for bone tissue engineering: current trends and forecasts for the future. J Biomed Mater Res. 2010;97A:514–35.
Jahan K, Tabrizian M. Composite biopolymers for bone regeneration enhancement in bony defects. Biomater Sci. 2016;4:25–39.
Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92.
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6.
Vacanti CA. History of tissue engineering and a glimpse into its future. Tissue Eng. 2006;12:1137–42.
Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, Tomsia AP. Bioactive glass in tissue engineering. Acta Biomater. 2011;7:2355–73.
Kaur G, Pandey OP, Singh K, Homa D, Scott B, Pickrell G. A review of bioactive glasses: their structure, properties, fabrication and apatite formation. J Biomed Mater Res A 2013; 254-74.
Hench LL, Splinter RJ, Allen WC, Greenlee TK. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1972;2:117–41.
Kaur G, Pickrell G, Sriranganathan N, Kumar V, Homa D. Review and state of the art: sol-gel and melt quenched bioactive glasses for tissue engineering. J Biomed Mater Res B Appl Biomater. 2016;104:1248–75.
Fu Q, Saiz E, Rahaman MN, Tomisia AP. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Mater Sci Eng C Mater Biol Appl. 2011;31:1245–56.
Brovarone C, Verne E, Robiglio L, Appendino P, Bassi F, Martinasso G, Muzio G, Canuto R. Acta Biomater. 2007;2:199–208.
Hench LL, Wilson J. Introduction to bioceramics. World scientific Singapore, Singapore 596224; 1993.
Liu X, Huang W, Fu H, Yao A, Wang D, Pan H, Lu WW. Bioactive borosilicate glass scaffolds: improvement on the strength of glass-based scaffolds for tissue engineering. J Mater Sci Mater Med. 2009;20:365–72.
Brovarone CV, Verné E, Appendino P. Macroporous bioactive glass-ceramic scaffolds for tissue engineering. J Mater Sci: Mater Med. 2006;17:1069–78.
Chen Q, Baino F, Spriano S, Pugno NM, Brovarone CV. Modelling of strength-porosity relationship in glass-ceramic foam scaffolds for bone repair. J Eur Ceram Soc. 2014;34:2663–73.
Brovarone CV, Miola M, Verné E. 3D glass ceramic scaffolds with antibacterial properties properties for bone grafting. Chem Eng J. 2008;137:129–36.
Salinas AJ, Román J, Regi MV, Oliveira JM, Correia RN, Fernandes MH. In vitro bioactivity of glass and glass ceramics of 3CaO.P2O5-CaO.SiO2-CaO.MgO.2SiO2 system. Biomaterials. 2000;21:251–7.
Murphy S, Boyd D, Moane S, Bennett M. The effect of composition on ion release from Ca–Sr–Na–Zn–Si glass bone grafts. J Mater Sci Mater Med. 2009;20:2028–35.
Aina V, Perardi A, Bergandi L, Malavasi G, Menabue L, Morterra C, Ghigo D. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblast. Chem Biol Interact. 2007;167:207–18.
Kaur G, Pickrell G, Kimsawatde G, Homa D, Allbee HA, Sriranganathan N. Synthesis, cytotoxicity, and hydroxyapatite formation in 27-Tris-SBF for sol gel based CaO-P2O5-SiO2-B2O3-ZnO bioactive glasses. Sci Rep. 2014;4:1–14.
Kaur G, Pandey OP, Chudasama BN, Kumar V. Combined and individual doxorubicin/vancomycin drug loading, release kinetics and apatite formation for the CaO – CuO – P2O5 – SiO2 – B2O5 mesoporous glasses. RSC Adv 2016; 51046–56.
Kaur G, Sharma P, Kumar V, Singh K. Assessment of in vitro bioactivity of SiO2 – BaO – ZnO – B2O3 – Al2O3 glasses: An Optico – analytical approach. Mater Sci and Engg. C 2012;32:1941–7.
Nielsens P. The biological role of strontium. Bone. 2004;35:583–8.
Lemaire H, et al. The calcium sensing receptor is involved in strontium ranelate induced osteoclast apoptosis. J Boil Chem. 2009;284:575–84.
Sriranganathan D, Kanwal N, Hing KA, Hill RG. Strontium substituted bioactive glasses for tissue engineered scaffolds: the importance of octacalcium phosphate. J Mater Sci: Mater Med. 2016;27:1–10.
Lao J, Nedelec JM, Edouard J. New strontium-based bioactive glasses: physiochemical reactivity and delivering capability of biologically active dissolution products. J Mater Chem. 2009;19:2940–9.
Neel EAA, Chrzanowski W, Pickup DM, O’Dell LA, Mordan NJ, Newport RJ, Smith ME, Knowles JC. Structure and properties of strontium-doped phosphate-based glasses. J R Soc Interface. 2009;6:435–46.
Gorustovich AA, Steimetz T, Cabrini RL, Porto Lopez JM. Osteoconductivity of strontium-doped bioactive glass particles: a histomorphometric study in rats. Soc Biomater. 2010;92A:232–7.
Pan H, Zhao XL, Zhang X, Zhang KB, Li LC, Li ZY, Lam WM, Lu WW, Wang DP, Huang WH, Lin KL, Chang J. Strontium borate glass: potential biomaterials for bone regeneration. J R Soc Interface. 2010;7:1025–31.
Nilihara K, Morena R, Hasselman DPH. Evaluation of KIc of brittle solids by the indentation method with low crack-to-indent ratios. J Mater Sci Lett. 1982;1:13–6.
Anstis GR, Chantikul R, Lawn BR, Marshall DB. A critical evaluation of indentation techniques for measuring fracture toughness: I, direct crack measurements. J Am Ceram Soc. 1981;64:533–8.
Adams JW, Ruh R, Mazdiyasni KS. Young's modulus, flexural strength, and fracture of yttria-stabilized zirconia versus temperature. J Am Ceram Soc. 1997;80:903–8.
Baik DS, No KS, Chun JS, Yoon YJ, Cho HY. A comparative method of machinability for mica based glass-ceramics. J Mater Sci. 1995;30:1801–6.
Baik DS, No KS, Chun JS, Cho HY. Effect of the aspect ratio of mica crystals and crystallinity on the microhardness and machinability of mica glass-ceramics. J Mater Process Technol. 1997;67:50–4.
Leon y, Leon CA. New perspectives in mercury porosimetry. Adv Colloid Interface Sci. 1998;76-77:341–72.
Kuboki Y, Takita H, Kobayashi D, Tsuruga E, Inoue M, Murata M, et al. BMP induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis. J Biomed Mater Res. 1998;39:190–9.
O’ Donnell MD, Watts SJ, Hill RG, Law RV. The effect of phosphate content on bioactivity of soda lime phosphosilicate glasses. J Mater Sci: Mater Med. 2009;20:1611–8.
Marsich L, Moimas L, Sergo V, Schmid C. Raman spectroscopic study of bioactive silica-based glasses: the role of alkali/alkali earth ratio on the non-bridging oxygen/bridging oxygen (NBO/BO) ratio. Spectroscopy. 2009;23:227–332.
Pemberton JE, Latifzadeh L. Raman spectroscopy of calcium phosphate glasses with varying CaO modifier concentrations. Chem Mater. 1991;3:195–200.
Moustafa YM, El-Egili K. Infrared spectra of sodium phosphate glasses. J Non- Cryst Solid. 1998;240:144–53.
Lu WH, Li KD, Lu CH, Teoh LG, Wu WH, Shen YC. Synthesis and characterization of mesoporous SiO2 – CaO – P2O5 bioactive glass by sol-gel process. Mater Trans. 2013;54:791–5.
Wu C, Fan W, Gelinsky M, Xiao Y, Simon P, Schulze R, Thomas D, Luo Y, Cuniberti G. Bioactive SrO-SiO2 glass with well-ordered mesopores: characterization, physiochemistry and biological properties. Acta Biomaterialia. 2011;7:1797–806.
Pereira MM, Clark AE, Hench LL. Effect on the rate of hydroxyapatite formation on Gel- silica surface. J Am Ceram Soc. 1995;78:2463–8.
Basu B. Toughening of Y-stabilized tetragonal zirconia ceramics. Int Mater Rev. 2005;50:239–56.
Bretcanu O, Chen Q, Misra SK, Boccaccini AR, Verne E, Vitale Brovarone C. Biodegradable polymer coated 45S5 bioglass derived glass-ceramic scaffolds for bone tissue engineering. Glass Tech: Eur J Glass Sci Tech A. 2007;48:227–34.
Mencik J. Strength and fracture of glass ceramics. Glass Sci and Tech 12 (1992). Amsterdam:Elsevier.
Fisher H, Schafer M, Marx R. Effect of surface roughness on flexural strength of veneer ceramics. J Dent Res. 2003;82:972–75.
Buttke TM, McCubrey JA, Owen TC. Use of an aqueous soluble tetrazolium:formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J Immunol Methods. 1993;157:233–40.
Berg K, Zhai L, Chen M, Kharazmi A, Owen TC. The use of a watersoluble formazan complex to quantitate the cell number and mitochondrial function of Leishmania major promastigotes. Parasitol Res. 1994;80:235–9.
Oudadesse H, Dietrich E, Gal YL, Pellen P, Bureau B, Mosrafa AA, Cathelineau G. Apatite forming ability and cytocompatibility of pure and Zn doped bioactive glasses. Biomed Mater. 2011;6:035006-5
Ranjan A, Pothayee N, Seleem MN, Tyler RD, Brenseke B, Sriranganathan N, Riffle JS, Kasimanickam R. Antibacterial efficacy of core-shell nanostructures encapsulating gentamicin against an in, vivo intracellular Salmonella model. Int J Nanomed 2009; 4:289–97.
Mandal B, Kundu S. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30:2956–65.
Lien SM, Ko LY, Huang TJ. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009;5:670–9.
Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83-A:105–15.
Garg S, Thakur S, Gupta A, Kaur G, Pandey OP. Antibacterial and anticancerous drug loading kinetics for (10-x) CuO-xZnO-20CaO-60SiO2-10P2O5 (2 ≤ x ≤ 8) mesoporous bioactive glasses. J Mater Sci: Mater Med. 2017;28:1–14.
Kaur G. Bioactive glasses: potential biomaterials for future therapy. Germany, Heidelberg: Springer, 2017.
Acknowledgements
The authors are thankful to Mr. Aayush Gupta for his valuable suggestions. One of the author GK is thankful to University Grant Commission (UGC) under the letter no. F 15/2013-2014/PDFWM – 2013-2014-GE – PUN – 14803 (SA-II) for providing financial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Thakur, S., Garg, S., Kaur, G. et al. Effect of strontium substitution on the cytocompatibility and 3-D scaffold structure for the xSrO–(10−x) MgO–60SiO2–20CaO–10 P2O5 (2 ≤ x ≤ 8) sol–gel glasses. J Mater Sci: Mater Med 28, 89 (2017). https://doi.org/10.1007/s10856-017-5901-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5901-z