Abstract
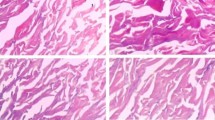
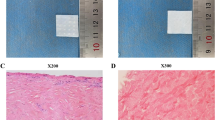
Acellular dermal matrix (ADM) has been widely used in repair and reconstruction of tissue defect. Therapeutic effect of porcine ADM (PADM) is inferior to that of human ADM (HADM). Relatively high immunogenicity and the resulting strong inflammatory response are major issue in application of PADM. We therefore treated reticular layer PADM (Rl-PADM) with matrix metalloproteinase-7 (MMP-7) and obtained a low-immunogenicity porcine dermal scaffold (LIPDS). Highly immunogenic components, tissue structure, cytocompatibility, and postgrafting histological changes of LIPDS were further investigated. Compared with Rl-PADM, LIPDS showed that the epithelial root sheath, cell debris, laminin, and type IV collagen were almost entirely removed, the structure remained normal, and the interfibrous space was relatively enlarged. Cytocompatibility of LIPDS was similar to that of HADM but superior to Rl-PADM. With regard to the extent of tissue ingrowth in terms of host fibroblasts infiltration and vascularization, LIPDS exhibited clear advantages over Rl-PADM after they had been subcutaneously transplanted in a rat model. In addition, no excessive inflammatory response was observed in LIPDS group up to 28 days postgraft, and the morphosis of collagenous fibers kept essentially normal. However, there were stronger inflammatory response and obvious collagen spallation in Rl-PADM group. The processes of integration and remodeling after the LIPDS grafting were similar to those of a normal wound healing response. The LIPDS graft was vascularized at a relatively high speed. Thus, as an implantable scaffold material, LIPDS is a superior template for guiding tissue regeneration and remodeling.










Similar content being viewed by others
References
Hrabchak C, Flynn L, Woodhouse KA. Biological skin substitutes for wound cover and closure. Expert Rev Med Devices. 2006;3:373–85.
Pereira C, Gold W, Herndon D. Review paper: burn coverage technologies: current concepts and future directions. J Biomater Appl. 2007;22:101–21.
Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–8.
Kinsella CR Jr, Grunwaldt LJ, Cooper GM, Mills MC, Losee JE. Scalp reconstruction: regeneration with acellular dermal matrix. J Craniofac Surg. 2010;21:605–7.
Capito AE, Tholpady SS, Agrawal H, Drake DB, Katz AJ. Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates. Ann Plast Surg. 2012;68:495–500.
Broderick G, McIntyre J, Noury M, Strom HM, Psoinos C, Christakas A, et al. Dermal collagen matrices for ventral hernia repair:comparative analysis in a rat model. Hernia. 2012;16:333–43.
Shaikh FM, Giri SK, Durrani S, Waldron D, Grace PA. Experience with porcine acellular dermal collagen implant in one-stage tension-free reconstruction of acute and chronic abdominal wall defects. World J Surg. 2007;31:1966–72.
Chavarriaga LF, Lin E, Losken A, Cook MW, Jeansonne LO, White BC, et al. Management of complex abdominal wall defects using acellular porcine dermal collagen. Am Surg. 2010;76:96–100.
Richter GT, Smith JE, Spencer HJ, Fan CY, Vural E. Histological comparison of implanted cadaveric and porcine dermal matrix grafts. Otolaryngol Head Neck Surg. 2007;137:239–42.
Ngo MD, Aberman HM, Hawes ML, Choi B, Gertzman AA. Evaluation of human acellular dermis versus porcine acellular dermis in an in vivo model for incisional henia repair. Cell Tissue Bank. 2011;12:135–45.
Campbell KT, Burns NK, Rios CN, Mathur AB, Butler CE. Human versus non-cross-linked porcine acellular dermal matrix used for ventral hernia repair: comparison of in vivo fibrovascular remodeling and mechanical repair strength. Plast Reconstr Surg. 2011;127:2321–32.
Chen RN, Ho HO, Tsai YT, Sheu MT. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25:2679–86.
Zhang J, Wang GY, Xiao YP, Fan LY, Wang Q. The biomechanical behavior and host response to porcine-derived small intestine submucosa, pericardium and dermal matrix acellular grafts in a rat abdominal defect model. Biomaterials. 2011;32:7086–95.
Prasertsung I, Kanokpanont S, Bunaprasert T, Thanakit V, Damrongsakkul S. Development of acellular dermis from porcine skin using periodic pressurized technique. J Biomed Mater Res B Appl Biomater. 2008;85:210–9.
DeSagun EZ, Botts JL, Srivastava A, Hanumadass M, Walter RJ. Long-Term outcome of xenogenic dermal matrix implantation in immunocompetent rats. J Surg Res. 2001;96:96–106.
Davis NF, McGuire BB, Callanan A, Flood HD, McGloughlin TM. Xenogenic extracellular matrices as potential biomaterials for interposition grafting in urological surgery. J Urol. 2010;184:2246–53.
Butler CE. The role of bioprosthetics in abdominal wall reconstruction. Clin Plast Surg. 2006;33:199–211.
Burns NK, Jaffari MV, Rios CN, Mathur AB, Butler CE. Non-cross-linked porcine acellular dermal matrices for abdominal wall reconstruction. Plast Reconstr Surg. 2010;125:167–76.
Thrailkill KM. Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35:1–10.
Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–36.
Zhang A, Song G, Cao C, Wang Y, Bian D. An experimental study on the attachment and growth of human skin fibroblast on a porcine collagenic dermal matrix in vitro. J Shandong Univ (Health Sciences). 2007;45:718–21.
Campbell KT, Burns NK, Ensor J, Butler CE. Metrics of cellular and vascular infiltration of human acellular dermal matrix in ventral hernia repairs. Plast Reconstr Surg. 2012;129:888–96.
Jiang J, Wu Y, Lu Q, Jia J, Shao Y, Song G. Effect of matrix metalloproteinase-7 on immunogenicity of porcine acellular dermal matrix. J Shandong Univ (Health Sciences). 2011;49:74–8.
The United States Pharmacopeial Convention. USP35-NF30. Biological tests/biological reactivity tests, in vitro. Rockville: The United States Pharmacopeial Convention; 2012. p. 92–4.
Walter RJ, Matsudab A, Reyes HM, Walter JM, Hanumadass M. Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burns. 1998;24:104–13.
Macleod TM, Williams G, Sanders R, Green CJ. Histological evaluation of Permacole as a subcutaneous implant over a 20-week period in the rat model. Br J Plast Surg. 2005;58:518–32.
Ayubi FS, Armstrong PJ, Mattia MS, Parker DM. Abdominal wall hernia repair: a comparison of Permacol and Surgisis grafts in a rat hernia model. Hernia. 2008;12:373–8.
Wong AK, Schonmeyr B, Singh P, Carlson DL, Li S, Mehrara BJ. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg. 2008;121:1144–52.
D’Souza SE, Ginsberg MK, Plow EF. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. TIBS. 1991;16:246–50.
Parrado AC, Canellada A, Gentile T, Rey-Roldán EB. Dopamine agonists upregulate IL-6 and IL-8 production in human keratinocytes. Neuroimmunomodulation. 2012;19:359–66.
Kolář M, Szabo P, Dvořánková B, Lacina L, Gabius HJ, Strnad H, et al. Upregulation of IL-6, IL-8 and CXCL-1 production in dermal fibroblasts by normal/malignant epithelial cells in vitro: immunohistochemical and transcriptomic analyses. Biol Cell. 2012;104:738–51.
Armour AD, Fish JS, Woodhouse KA, Semple JL. A comparison of human and porcine acellularized dermis: interactions with human fibroblasts in vitro. Plast Reconstr Surg. 2006;117:845–56.
Acknowledgments
This study was supported in part by grants from Building Project on National Clinical Key Specialty of China ([2012]649), Science and Technology Development Project of Shangdong Province (2013GSF11870), Science and Technology Development Project of Shangdong Province Health and Medicine (2011HZ008), and Business Project of Study Abroad Returnees in Jinan (20080405).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, G., Wu, Y., Wang, F. et al. Development and preparation of a low-immunogenicity porcine dermal scaffold and its biocompatibility assessment. J Mater Sci: Mater Med 26, 170 (2015). https://doi.org/10.1007/s10856-015-5503-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5503-6