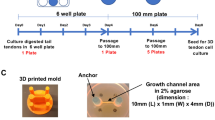
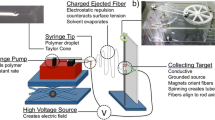
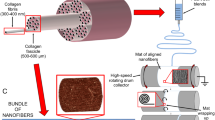
Abstract
Development of tissue engineering scaffolds relies on careful selection of pore architecture and chemistry of the cellular environment. Repair of skeletal soft tissue, such as tendon, is particularly challenging, since these tissues have a relatively poor healing response. When removed from their native environment, tendon cells (tenocytes) lose their characteristic morphology and the expression of phenotypic markers. To stimulate tendon cells to recreate a healthy extracellular matrix, both architectural cues and fibrin gels have been used in the past, however, their relative effects have not been studied systematically. Within this study, a combination of collagen scaffold architecture, axial and isotropic, and fibrin gel addition was assessed, using ovine tendon-derived cells to determine the optimal strategy for controlling the proliferation and protein expression. Scaffold architecture and fibrin gel addition influenced tendon cell behavior independently in vitro. Addition of fibrin gel within a scaffold doubled cell number and increased matrix production for all architectures studied. However, scaffold architecture dictated the type of matrix produced by cells, regardless of fibrin addition. Axial scaffolds, mimicking native tendon, promoted a mature matrix, with increased tenomodulin, a marker for mature tendon cells, and decreased scleraxis, an early transcription factor for connective tissue. This study demonstrated that both architectural cues and fibrin gel addition alter cell behavior and that the combination of these signals could improve clinical performance of current tissue engineering constructs.





Similar content being viewed by others
References
Kastelic J, Galeski A, Baer E. Multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11–23.
Biewener A. Tendons and ligaments: structure, mechanical behavior and biological function. In: Fratzl P, editor. Collagen. New York: Springer; 2008. p. 269–84.
Amiel D, Frank C, Harwood F, Fronek J, Akeson W. Tendons and ligaments a morphological and biochemical comparison. J Orthop Res. 1984;1(3):257–65.
Klatte-Schulz F, Pauly S, Scheibel M, Greiner S, Gerhardt C, Schmidmaier G, Wildemann B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur Cells Mater. 2012;24:74–89.
Bayer M, Yeung C-YC, Kadler KE, Qvortrup K, Baar K, Svensson RB, Magnusson SP, Krogsgaard M, Koch M, Kjaer M. The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials. 2010;31(18):4889–97.
Caliari SR, Harley BAC. The effect of anisotropic collagen-gag scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials. 2011;32(23):5330–40.
Kapacce Z, Richardson SH, Lu Y, Starborg T, Holmes DF, Baar K, Kadler KE. Tension is required for fibripositor formation. Matrix Biol. 2008;27(4):371–5.
Kalson NS, Holmes DF, Kapacee Z, Otermin I, Lu Y, Ennos RA, Canty-Laird EG, Kadler KE. An experimental model for studying the biomechanics of embryonic tendon: evidence that the development of mechanical properties depends on the actinomyosin machinery. Matrix Biol. 2010;29(8):678–89.
Smithmyer ME, Sawickia LA, Kloxin AM. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater Sci. 2014;2:634–50.
Ross JJ, Tranquillo R. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22(6):477–90.
Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011;32(31):7856–69.
Lohse N, Schulz J, Schliephake H. Effect of fibrin on osteogenic differentiation and VEGf expression of bone marrow stromal cells in mineralized scaffolds: a three-dimensional analysis. Eur Cells Mater. 2012;23:413–24.
Pawelec KM, Husmann A, Best SM, Cameron RE. Understanding anisotropy and architecture in ice-templated biopolymer scaffolds. Mater Sci Eng. 2014;37:141–7.
Pawelec KM, Husmann A, Best SM, Cameron RE. A design protocol for tailoring ice-templated scaffold structure. J R Soc Interface. 2014;11:20130958.
Davidenko N, Gibb T, Schuster C, Best SM, Campbell JJ, Watson CJ, Cameron RE. Biomimetic collagen scaffolds with anisotropic pore architecture. Acta Biomater. 2012;8(2):667–76.
Damink L, Dijkstra PJ, van Luyn MJA, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17(8):765–73.
Kim Y-J, Sah RL, Doong J-YH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174(1):168–76.
Farndale R, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochem Biophys Acta. 1986;883(2):173–7.
Venugopal J, Ma LL, Yong T, Ramakrishna S. In vitro study of smooth muscle cells on polycaprolactone and collagen nanofibrous matrices. Cell Biol Int. 2005;29:861–7.
Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298(1):234–47.
Pearlstein E, Gold LI, Garciapardo A. Fibronectin—review of its structure and biological-activity. Mol Cell Biochem. 1980;29(2):103–28.
Friess W. Collagen—biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113–36.
Ayad S, Boot-Handford RP, Humphries MJ, Kadler KE, Shuttleworth CA. The extracellular matrix facts book. 2nd ed. New York: Academic Press; 1998.
Schneider PRA, Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J Orthop Res. 2011;29(9):1351–60.
Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis—a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121(4):1099–110.
Carlberg AL, Tuan RS, Hall DJ. Regulation of scleraxis function by interaction with the BHLH protein E47. Mol Cell Biol Res Commun. 2000;3(2):82–6.
Taylor SE, Vaughan-Thomas A, Clements DN, Pinchbeck G, Macrory LC, Smith RKW, Clegg PD. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord. 2009;10(1):27.
Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012;30(4):606–12.
Yamana K, Wada H, Takahashi Y, Sato H, Kasahara Y, Kiyoki M. Molecular cloning and characterization of Chm1 l, a novel membrane molecule similar to chondromodulin-I. Biochem Biophys Res Commun. 2001;280(4):1101–6.
Qi J, Dmochowski JM, Banes AN, Tsuzaki M, Bynum D, Patterson M, Creighton A, Gomez S, Tech K, Cederlund A, Banes AJ. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol. 2012;113(6):861–71.
Kishore V, Uquillas JA, Dubikovsky A, Alshehabat MA, Snyder PW, Breur GJ, Akkus O. In vivo response to electrochemically aligned collagen bioscaffolds. J Biomed Mater Res, Part B. 2012;100B(2):400–8.
Yin Z, Chen X, Chen JL, Shen WL, Nguyen TMH, Gao L, Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31(8):2163–75.
Caliari SR, Weisgerber DW, Ramirez MA, Kelkhoff DO, Harley BAC. The influence of collagen-glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J Mech Behav Biomed Mater. 2012;11:27–40.
Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the alamar blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997;204(2):205–8.
Mazzocca AD, Chowaniec D, McCarthy MB, Beitzel K, Cote MP, McKinnon W, Arciero R. In vitro changes in human tenocyte cultures obtained from proximal biceps tendon: multiple passages result in changes in routine cell markers. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1666–72.
Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development. 2001;128(19):3855–66.
Wang YZ, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26(34):7082–94.
Kishore V, Bullock W, Sun X, Van Dyke WS, Akkus O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials. 2012;33(7):2137–44.
Acknowledgments
The authors gratefully acknowledge the financial support of the Gates Cambridge Trust, the ERC Advanced Grant 320598 3D-E, and the Technology Strategy Board UK. Special thanks to Nigel Loveridge for help with the statistical analysis and Natalia Davidenko for supplying the freeze drying molds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pawelec, K.M., Wardale, R.J., Best, S.M. et al. The effects of scaffold architecture and fibrin gel addition on tendon cell phenotype. J Mater Sci: Mater Med 26, 13 (2015). https://doi.org/10.1007/s10856-014-5349-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-014-5349-3