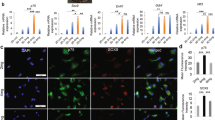
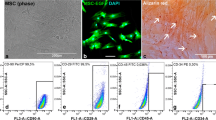
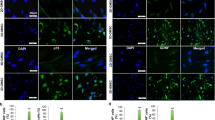
Abstract
The development of artificial off-the-shelf conduits that facilitate effective nerve regeneration and recovery after repair of traumatic nerve injury gaps is of fundamental importance. Collagen–glycosaminoglycan (GAG) matrix mimicking Schwann cell (SC) basal lamina has been proposed as a suitable and biologically rational substrate for nerve regeneration. In the present study, we have focused on the permissiveness of this matrix type for SC migration and repopulation, as these events play an essential role in nerve remodeling. We have also demonstrated that SCs cultured within collagen–GAG matrix are compatible with non-viral dendrimer-based gene delivery, that may allow conditioning of matrix-embedded cells for future gene therapy applications.





Similar content being viewed by others
References
Noble J, Munro CA, Prasad VSSV, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–22.
Millesi H, Meissl G, and Berger A The interfasicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972; 54-A:727-750.
Belkas J, Shoichet MS, Midha R. Axonal guidance channels in peripheral nerve regeneration. Oper Tech Orthop. 2005;14:190–8.
Raheja A, Suri V, Suri A, Sarkar C, Srivastava A, Mohanty S, Jain KG, Sharma MC, Mallick HN, Yadav PK, Kalaivani M, Pandey RM. Dose-dependent facilitation of peripheral nerve regeneration by bone marrow-derived mononuclear cells: a randomized controlled study: laboratory investigation. J Neurosurg. 2012;117:1170–81.
Shakhbazau A, Martinez JA, Xu QG, Kawasoe J, Van Minnen MJ, Midha R. Evidence for a systemic regulation of neurotrophin synthesis in response to peripheral nerve injury. J Neurochem. 2012;122:501–11.
Singh B, Xu QG, Franz CK, Zhang R, Dalton C, Gordon T, Verge VM, Midha R, Zochodne DW. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm. J Neurosurg. 2012;116:498–512.
Yang DY, Sheu ML, Su HL, Cheng FC, Chen YJ, Chen CJ, Chiu WT, Yiin JJ, Sheehan J, Pan HC. Dual regeneration of muscle and nerve by intravenous administration of human amniotic fluid-derived mesenchymal stem cells regulated by stromal cell-derived factor-1alpha in a sciatic nerve injury model. J Neurosurg. 2012;116:1357–67.
Yang Y, De LL, Rives CB, Jang JH, Lin WC, Shull KR, Shea LD. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104:433–46.
Pabari A, Yang SY, Mosahebi A, Seifalian AM. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release. 2011;156:2–10.
Tan A, Rajadas J, Seifalian AM. Biochemical engineering nerve conduits using peptide amphiphiles. J Control Release. 2012;163:342–52.
Sun M, McGowan M, Kingham PJ, Terenghi G, Downes S. Novel thin-walled nerve conduit with microgrooved surface patterns for enhanced peripheral nerve repair. J Mater Sci Mater Med. 2010;21:2765–74.
Chen X, Yang Y, Yao J, Lin W, Li Y, Chen Y, Gao Y, Yang Y, Gu X, Wang X. Bone marrow stromal cells-loaded chitosan conduits promote repair of complete transection injury in rat spinal cord. J Mater Sci Mater Med. 2011;22:2347–56.
Zheng L, Cui HF. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci Mater Med. 2012;23:2291–302.
Mobasseri SA, Terenghi G, Downes S. Micro-structural geometry of thin films intended for the inner lumen of nerve conduits affects nerve repair. J Mater Sci Mater Med. 2013;24:1639–47.
Kehoe S, Zhang XF, Boyd D. Composition–property relationships for an experimental composite nerve guidance conduit: evaluating cytotoxicity and initial tensile strength. J Mater Sci Mater Med. 2011;22:945–59.
Yahyouche A, Zhidao X, Triffitt JT, Czernuszka JT, Clover AJ. Improved angiogenic cell penetration in vitro and in vivo in collagen scaffolds with internal channels. J Mater Sci Mater Med. 2013;24:1571–80.
Steele TW, Huang CL, Nguyen E, Sarig U, Kumar S, Widjaja E, Loo JS, Machluf M, Boey F, Vukadinovic Z, Hilfiker A, Venkatraman SS. Collagen–cellulose composite thin films that mimic soft-tissue and allow stem-cell orientation. J Mater Sci Mater Med. 2013;24:2013–27.
Archibald SJ, Shefner J, Krarup C, Madison RD. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci. 1995;15:4109–23.
Kemp SWP, Syed S, Walsh SK, Zochodne DW, Midha R. Collagen nerve conduits promote enhanced axonal regeneration, Schwann cell association, and neovascularization compared to silicone conduits. Tissue Eng A. 2009;15(8):1975–88.
Archibald SJ, Krarup C, Shefner J, Li ST, Madison RD. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991;306:685–96.
Mahmood A, Wu H, Qu C, Xiong Y, Chopp M. Effects of treating traumatic brain injury with collagen scaffolds and human bone marrow stromal cells on sprouting of corticospinal tract axons into the denervated side of the spinal cord. J Neurosurg. 2013;118:381–9.
Aronson JP, Mitha AP, Hoh BL, Auluck PK, Pomerantseva I, Vacanti JP, Ogilvy CS. A novel tissue engineering approach using an endothelial progenitor cell-seeded biopolymer to treat intracranial saccular aneurysms. J Neurosurg. 2012;117:546–54.
Hudson AR, Morris J, Weddell G, Drury A. Peripheral nerve autografts. J Surg Res. 1972;12:267–74.
Zhao Q, Dahlin LB, Kanje M, Lundborg G. The formation of a ‘pseudo-nerve’ in silicone chambers in the absence of regenerating axons. Brain Res. 1992;592:106–14.
Richardson PM. Neurotrophic factors in regeneration. Curr Opin Neurobiol. 1991;1:401–6.
Brushart TME. In: Gelberman RH, editor. Operative nerve repair and reconstruction. Philadelphia: J.B. Lippincott; 1991. p. 215–30.
Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol. 1994;23:1–28.
Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116.
Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005;64:613–22.
Lavdas AA, Franceschini I, Dubois-Dalcq M, Matsas R. Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia. 2006;53:868–78.
Hu Y, Leaver SG, Plant GW, Hendriks WT, Niclou SP, Verhaagen J, Harvey AR, Cui Q. Lentiviral-mediated transfer of CNTF to Schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol Ther. 2005;11:906–15.
Bachelin C, Zujovic V, Buchet D, Mallet J, Baron-Van EA. Ectopic expression of polysialylated neural cell adhesion molecule in adult macaque Schwann cells promotes their migration and remyelination potential in the central nervous system. Brain. 2010;133:406–20.
Weidner N, Blesch A, Grill RJ, Tuszynski MH. Nerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1. J Comp Neurol. 1999;413:495–506.
Walsh S, Biernaskie J, Kemp SW, Midha R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097–107.
Komiyama T, Nakao Y, Toyama Y, Asou H, Vacanti CA, Vacanti MP. A novel technique to isolate adult Schwann cells for an artificial nerve conduit. J Neurosci Methods. 2003;122:195–200.
Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933–7.
Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat Methods. 2006;3:109–16.
Shakhbazau A, Shcharbin D, Petyovka N, Goncharova N, Seviaryn I, Kosmacheva S, Bryszewska M, Potapnev M. Non-virally modified human mesenchymal stem cells produce ciliary neurotrophic factor in biodegradable fibrin-based 3D scaffolds. J Pharm Sci. 2012;101:1546–54.
Parrinello S, Napoli I, Ribeiro S, Wingfield DP, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–55.
Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–42.
Tohill MP, Mann DJ, Mantovani CM, Wiberg M, Terenghi G. Green fluorescent protein is a stable morphological marker for Schwann cell transplants in bioengineered nerve conduits. Tissue Eng. 2004;10:1359–67.
Weber N, Ortega P, Clemente MI, Shcharbin D, Bryszewska M, de la Mata FJ, Gomez R, Munoz-Fernandez MA. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J Control Release. 2008;132:55–64.
Shcharbin D, Pedziwiatr E, Nowacka O, Kumar M, Zaborski M, Ortega P, de la Mata FJ, Gomez R, Munoz-Fernandez MA, Bryszewska M. Carbosilane dendrimers NN8 and NN16 form a stable complex with siGAG1. Colloids Surf B. 2011;83:388–91.
Shakhbazau A, Shcharbin D, Bryszewska M, Kumar R, Wobma HM, Kallos MS, Goncharova N, Seviaryn I, Kosmacheva S, Potapnev M, Midha R. Non-viral engineering of skin precursor-derived Schwann cells for enhanced NT-3 production in adherent and microcarrier culture. Curr Med Chem. 2012;19:5572–9.
Santos JL, Oramas E, Pego AP, Granja PL, Tomas H. Osteogenic differentiation of mesenchymal stem cells using PAMAM dendrimers as gene delivery vectors. J Control Release. 2009;134:141–8.
Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59(4):740–7.
Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ. Laminin activates NF-kappaB in Schwann cells to enhance neurite outgrowth. Neurosci Lett. 2008;439:42–6.
Webber C, Zochodne D. The nerve regenerative microenvironment: early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51–9.
Lee JY, Giusti G, Friedrich PF, Archibald SJ, Kemnitzer JE, Patel J, Desai N, Bishop AT, Shin AY. The effect of collagen nerve conduits filled with collagen–glycosaminoglycan matrix on peripheral motor nerve regeneration in a rat model. J Bone Jt Surg Am. 2012;94:2084–91.
Chamberlain LJ, Yannas IV, Hsu HP, Strichartz G, Spector M. Collagen–GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp Neurol. 1998;154:315–29.
Chamberlain LJ, Yannas IV, Hsu HP, Strichartz GR, Spector M. Near-terminus axonal structure and function following rat sciatic nerve regeneration through a collagen–GAG matrix in a ten-millimeter gap. J Neurosci Res. 2000;60:666–77.
Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008;95:4013–24.
Zochodne D. Neurobiology of peripheral nerve regeneration. 1st ed. New York: Cambridge University Press; 2008.
Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–71.
Pieper JS, van Wachem PB, van Luyn MJA, Brouwer LA, Hafmans T, Veerkamp JH, van Kuppevelt TH. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials. 2000;21:1689–99.
Liu J, Chau CH, Liu H, Jang BR, Li X, Chan YS, Shum DK. Upregulation of chondroitin 6-sulphotransferase-1 facilitates Schwann cell migration during axonal growth. J Cell Sci. 2006;119:933–42.
Cragnolini AB, Friedman WJ. The function of p75NTR in glia. Trends Neurosci. 2008;31:99–104.
Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–33.
Taniuchi M, Clark HB, Johnson EM Jr. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci USA. 1986;83:4094–8.
Notterpek L. Neurotrophins in myelination: a new role for a puzzling receptor. Trends Neurosci. 2003;26:232–4.
Khursigara G, Bertin J, Yano H, Moffett H, DiStefano PS, Chao MV. A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. J Neurosci. 2001;21:5854–63.
Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–8.
Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–93.
Fernandez-Valle C, Gorman D, Gomez AM, Bunge MB. Actin plays a role in both changes in cell shape and gene-expression associated with Schwann cell myelination. J Neurosci. 1997;17:241–50.
Bentley CA, Lee KF. p75 is important for axon growth and Schwann cell migration during development. J Neurosci. 2000;20:7706–15.
Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci USA. 1994;91:2795–9.
Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, MacKenzie KF, Klussmann E, Lynch MJ, Sikorski SL, Nuriel T, Tsigelny I, Zhang J, Houslay MD, Chao MV, Akassoglou K. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–32.
Gordon T, Brushart TM, Amirjani N, Chan KM. The potential of electrical stimulation to promote functional recovery after peripheral nerve injury—comparisons between rats and humans. Acta Neurochir Suppl. 2007;100:3–11.
Bianchi BR, Zhang XF, Reilly RM, Kym PR, Yao BB, Chen J. Species comparison and pharmacological characterization of human, monkey, rat and mouse TRPA1 channels. J Pharmacol Exp Ther. 2012;341(2):360–8.
Harries LW, Brown JE, Gloyn AL. Species-specific differences in the expression of the HNF1A, HNF1B and HNF4A genes. PLoS ONE. 2009;4:e7855.
Holden PR, Tugwood JD. Peroxisome proliferator-activated receptor alpha: role in rodent liver cancer and species differences. J Mol Endocrinol. 1999;22:1–8.
Shakhbazau A, Shcharbin D, Seviaryn I, Goncharova N, Kosmacheva S, Potapnev M, Bryszewska M, Kumar R, Biernaskie J, Midha R. Dendrimer-driven neurotrophin expression differs in temporal patterns between rodent and human stem cells. Mol Pharm. 2012;9:1521–8.
Gassmann K, Abel J, Bothe H, Haarmann-Stemmann T, Merk HF, Quasthoff KN, Rockel TD, Schreiber T, Fritsche E. Species-specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ Health Perspect. 2010;118:1571–7.
Smith AM, Dragunow M. The human side of microglia. Trends Neurosci. 2014;37:125–35.
Steffenhagen C, Kraus S, Dechant FX, Kandasamy M, Lehner B, Poehler AM, Furtner T, Siebzehnrubl FA, Couillard-Despres S, Strauss O, Aigner L, Rivera FJ. Identity, fate and potential of cells grown as neurospheres: species matters. Stem Cell Rev. 2011;7:815–35.
Granowitz C, Berkowitz RD, Goff SP. Mutations affecting the cytoplasmic domain of the Moloney murine leukemia virus envelope protein: rapid reversion during replication. Virus Res. 1996;41:25–42.
Taddeo B, Carlini F, Verani P, Engelman A. Reversion of a human immunodeficiency virus type 1 integrase mutant at a second site restores enzyme function and virus infectivity. J Virol. 1996;70:8277–84.
Kang HC, Huh KM, Bae YH. Polymeric nucleic acid carriers: current issues and novel design approaches. J Control Release. 2012;164:256–64.
Dandekar P, Jain R, Keil M, Loretz B, Muijs L, Schneider M, Auerbach D, Jung G, Lehr CM, Wenz G. Cellular delivery of polynucleotides by cationic cyclodextrin polyrotaxanes. J Control Release. 2012;164:387–93.
Martello F, Piest M, Engbersen JF, Ferruti P. Effects of branched or linear architecture of bioreducible poly(amido amine)s on their in vitro gene delivery properties. J Control Release. 2012;164:372–9.
Bonner DK, Zhao X, Buss H, Langer R, Hammond PT. Crosslinked linear polyethylenimine enhances delivery of DNA to the cytoplasm. J Control Release. 2013;167:101–7.
Tomalia DA. Dendrimer research. Science. 1991;252:1231.
Pandita D, Santos JL, Rodrigues J, Pego AP, Granja PL, Tomas H. Gene delivery into mesenchymal stem cells: a biomimetic approach using RGD nanoclusters based on poly(amidoamine) dendrimers. Biomacromolecules. 2011;12:472–81.
Shakhbazau AV, Shcharbin DG, Goncharova NV, Seviaryn IN, Kosmacheva SM, Kartel NA, Bryszewska M, Majoral JP, Potapnev MP. Neurons and stromal stem cells as targets for polycation-mediated transfection. Bull Exp Biol Med. 2011;151:126–9.
Acknowledgments
This research was supported by CIHR Grant #163322, by the Center for Excellence in Nerve Regeneration (partnership between the Hotchkiss Brain Institute, University of Calgary and Integra LifeSciences), and by Alberta Innovates-Health Solutions (fellowship support for A.S.). The authors thank Ranjan Kumar and Indranil Dey for their kind assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shakhbazau, A., Archibald, S.J., Shcharbin, D. et al. Aligned collagen–GAG matrix as a 3D substrate for Schwann cell migration and dendrimer-based gene delivery. J Mater Sci: Mater Med 25, 1979–1989 (2014). https://doi.org/10.1007/s10856-014-5224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5224-2