Abstract
In this work a modified approach to improve the capabilities of nanosphere lithography was performed to obtain ordered arrays of gold nanopyramids that can be used as Surface-Enhanced Raman Scattering (SERS) substrates. Specifically, shadow sphere lithography in combination with nanosphere lithography was used to fabricate metallic nanostructures of different sizes starting from the same mask of silica microspheres. An extensive study of the obtained samples with scanning electron microscopy, atomic force microscopy and Raman characterizations confirms that indeed this modification of the standard nanosphere lithography is an excellent candidate to produce ordered arrays of Au nanopyramids with good control on their geometric properties. Rhodamine 6G dye was used as a probe molecule to test our SERS substrates. These features can be used in turn to optimize the SERS substrates for several potential applications. Remarkably, it is shown that by this simple technique one can achieve enhancement factors and detection capabilities of dyes that are comparable with those obtained by using more sophisticated techniques as proposed recently in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, nanomaterials play a central role in the research and fabrication of new materials, with numerous applications ranging from physical to biological sciences, and from medicine to everyday technology [1]. Much of the recent research is focused on developing and testing new techniques for the fabrication of nanostructures and devices that would ideally guarantee the following characteristics: easy to implement, highly precise, reusable, and of low cost. For this reason, over the years a number of techniques have been developed, such as spray pyrolysis, chemical vapor deposition, ion beam synthesis, pulsed-laser deposition and so on [2].
One of the techniques that have received greater attention is lithography [3]. Indeed, there exist several types of lithographic techniques, such as optical lithography, electron beam lithography, X-ray lithography, nanosphere lithography (NSL) and shadow sphere lithography (SSL) [4,5,6,7,8].
Standard NSL makes use of nano/micro spherical colloidal particles (polymers, latex or silica), monodisperse, to form a compact ordered monolayer following a honeycomb pattern. This particle monolayer constitutes a lithographic mask that can be used in combination with thermal evaporation deposition or ion implantation to produce periodic nanostructures. A fine control of the lithographic mask interstices would allow the tunability of the size, shape and periodic spacing of the nanostructure [9]. Standard NSL is very rigid in the sense that as the lithographic mask is made of spheres, one can typically obtain only pyramidal nanostructures related to the mask interstices. Moreover, once the mask is chosen, there is no possibility to vary the shape and size of these structures. Therefore if one needs to vary the geometry of the nanostructures, it is advisable to use some of the modifications of the standard NSL, which we collectively call here “modified nanosphere lithography”.
SSL is one of the most successful modifications of NSL, due to the fact that it is easy-to-implement and allows for a precise control of the final geometry. Indeed, all is needed is to change the angle at which the substrate with the mask is placed during the thin film evaporation process. It is for this reason that since its conception [10], it has acquired great importance over the years, and it is now considered as one of the most promising methods for the discovery of new materials [11,12,13,14,15,16,17,18,19]. Even though NSL has been widely utilized, its reproducibility, stability and scalability still remain a challenge and are currently of interest for the scientific community. Regarding the spin coating method, it was reported in [20] that a low rotation speed leads to a surface with multilayer spheres and, on the other hand, a high rotation speed results in areas without spheres [21]. Hence, the importance to study the NSL process and its variants, which requires a large experimental analysis for different sizes of nanospheres, speed rotation, time, drop method, and so on [20].
Clearly, the technique to be used for NSL is related to the particular application that one has in mind. In our case, the aim is to prepare substrates with ordered arrays of metallic nanoparticles on the surface for their use as detectors of organic molecules, such as dyes. Indeed, Rhodamine dyes are regularly used as probe molecules to demonstrate the Surface-Enhanced Raman Scattering (SERS) effect on different nanostructured substrates. One of the main advantages of SERS is that it allows the detection of molecules in parts per billion (ppb) [22]. For this reason, several applications in biological, medical and pharmaceutical sciences have been reported [23]. An important observation in this context is the fact that the greater the surface area of the nanoparticles, the stronger is the SERS effect [24]. Therefore, a fundamental problem in the design of nanoparticles is the development of techniques capable of maximizing the so-called “active area” of the nanoparticles.
For all these reasons, this work is devoted to a comparison of SSL and NSL regarding the fabrication of Au nanopyramids, with a particular focus on their use as SERS substrates [25]. After an extensive analysis of the obtained Au nanoparticles by means of Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM), and Raman spectroscopy, we conclude that both techniques are suitable for the fabrication of SERS substrates. The use of one over the other may therefore depend on other factors, most importantly the molecule of interest in the study. Nevertheless, the fact that SSL is easier to perform and control makes it a better candidate for modified nanosphere lithography. In particular, the combined process of inclined ther- mal evaporation with NSL allows us to generate ordered arrays of Au nanopyramids with different shapes and sizes over silicon substrates. We demonstrate that the Au nanopyramids are suitable as SERS substrates for the Rhodamine 6G (R6G) detection. Indeed, it is remarkable that, although no free-parameter (angle) optimization has been performed, and no systematic investigation on the detection limits was included, the results show that our enhancement factors are comparable with those obtained recently by using more sophisticated methods [26]. Moreover, our SERS substrates are potentially capable to detect dyes such as R6G up to limits that are in line with some of the state-of-the-art techniques in the literature [27]. Finally, these ordered arrays can lead to homogeneous SERS response for position-independent measurements.
2 Experimental setup
2.1 Materials and methods
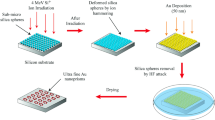
Spherical sub-micrometer-sized silica particles (SiO2 particles) were fabricated by a modified Stöber process [28, 29] using chemical reagents from Sigma-Aldrich and J.T. Baker. For this synthesis, first we prepared a mixture of 8 mL of ammonium hydroxide [NH4OH, with 28–30% purity, molecular weight 35.05 g/mol and density 0.99 g/cm3] and 5.5 mL of distilled water [molecular weight 18 g/mol]. A second mixture is prepared via magnetic stirring of 1.8 mL of tetraethyl orthosilicate [TEOS, Si(OC2H5)4 with 99% purity, molecular weight 208.33 g/mol, density of 0.93 g/cm3 and boiling point of 163–167 °C] and 20 mL of ethanol [EtOH, CH3CH2OH, with ≥ 99.5% purity, molecular weight of 46.07 g/mol, density of 0.79 g/cm3, boiling point of 78 °C]. Finally, the first mixture is added to the second one by slowly dripping.
The synthesis is carried out at a temperature between 12 °C and 16 °C under constant magnetic stirring and is left to react for at least 90 min.
Once obtained the silica particles in solution, the next step is to fabricate the lithographic masks. For this, the solution is deposited on Si substrates by means of a spin coater system. Si (111) wafers were previously cut into square pieces of 1 × 1 cm2, and a cleaning treatment called “the piranha solution” is applied. This consists of a mixture of sulfuric acid [H2SO4, purity 95–98%, molecular weight 98.08 g/mol, density 1.84 g/cm3, boiling point 290 °C] and hydrogen peroxide [H2O2, Sigma-Aldrich, 30% purity, molecular weight 34.01 g/mol] in a ratio of 3:1, which are left to react for 30 min under magnetic stirring and subsequently they are rinsed in Milli-Q ultrapure water.
For the deposition of the silica particles in a compact monolayer on silicon substrates, the Spin coating technique is used [spin coater model G3 from Specialty Coating Systems]. The process is performed at a temperature of 17 °C, with humidity of 50%. The colloidal solution was spin coated at a speed of 500 rpm for 20 s, followed by a second step at 1000 rpm for 200 s.
Finally, a Au thin film is deposited on the monolayer mask of SiO2 particles over the Si substrate. For this an e-gun thermal evaporation system was used. The thickness of the Au film was estimated using an oscillating quartz crystal. A 50 nm thick thin film was deposited on the silica monolayer and on the bare silicon substrate (for comparison purposes). The evaporation parameters were: a 4 kV voltage, a vacuum pressure of 8.8 × 10−6 Torr, a beam current in the 10–100 mA range and a deposition rate of 0.7 Ȧ/s. After the evaporation process, the lithographic silica mask was completely removed by keeping the samples in an ultrasonic bath for 25 min.
2.2 NSL and SSL
One of the limitations of nanosphere lithography (NSL) as a technique for the fabrication of nanostructures is the fact that when the size of the nano/micro spheres that form the mask is fixed, then the geometric pattern and the size of the corresponding nanostructure is also fixed. Indeed, when one evaporates the thin film, the pattern is formed following the mask interstices. The most direct way to change the size of the nanostructures is therefore to vary the diameter of the particles used for the mask. However, this requires the elaboration of new silica particles and of a new mask. Moreover, having a very precise control over the particles’ sizes is not easy with the current techniques.
A very efficient method to modify the size of the nanostructures avoiding the change of the lithographic mask is the so-called shadow sphere lithograhy (SSL), which consists in varying the evaporation angle of the material (see Fig. 1).
2.3 Characterization
The size, size distribution and shape of the SiO2 particles on the Si substrate were studied with SEM. The array of Au nanoparticles and their sizes were studied with SEM and AFM. SEM measurements were performed using secondary electrons with an energy of 5 kV using a JSM-7800F JEOL microscope. The tapping mode AFM studies were carried out in air by using a NT-MDT Solver Next equipment. Silicon cantilevers with a tip curvature radius of about 10 nm were used.
In order to detect and quantify the SERS effect in these pyramidal nanoparticles (NPs), the Rhodamine 6G dye molecule (R6G, C28H31N2O3 with 95% purity) was used [30]. For this, the substrates were first dipped into an aqueous stock solution of R6G at three different concentrations: 10−5 M, 10−6 M and 10−7 M for 24 h, without rinsing, and then they were left to dry at room temperature. The Raman-SERS spectra were measured using a micro-Raman NTEGRA Spectra instrument from NT-MDT with a 100 × objective and laser excitation at 532 nm. The exposure time was 10 s and the CCD was cooled down to −70 °C, in order to obtain the spectrum in the mode of single acquisition.
3 Results and discussion
3.1 SEM and AFM analysis
Monolayer arrays. Once the close-packed monolayer arrays of SiO2 particles are obtained, these are used as lithographic masks. Figure 2 shows representative SEM images of the modified lithographic masks that have been used for this work: Figs. 2a and b show the first new mask, hereafter labelled as “Mask1”, which is the one used for NSL and SSL; Figs. 2c and d show the second new mask, hereafter labelled as “Mask0”, which is the one used for NSL. These masks were mapped with SEM in different regions in order to observe the extension and homogeneity of the monolayer array of silica particles. When studying these samples using SEM, it is fundamental to focus on two factors that guarantee a good efficiency of the masks for their use in nanolithography: one is the homogeneity in shape (spherical) and size (monodisperse) of the silica particles, and the other is the close-packed array of the spheres. Figures 2a and c show monolayer regions exhibiting long-range order and spatial homogeneity with minimal defects. By performing an analysis with the ImageJ software [31], the average diameter of the silica particles was determined: 650 ± 23 nm for Mask1 and 465 ± 24 nm for Mask0. More details on the formation and characterization of similar masks have been reported in [32].
SEM images of the new lithographic masks (top line for Mask1 and bottom line for Mask0) at different magnifications: a and c 2000 × and scale bar 10 µm, in order to observe the extension of the array; b and d 10,000 × and scale bar 1 µm, in order to observe in detail the spheres and their close-packed array
NSL. Here we report a comparison between the Au NPs obtained by evaporation using Mask1 and the Au NPs obtained using Mask0. Correspondingly, the former NPs will be labelled as “AuNP1”, while the latter will be labelled as “AuNP0”.
Figure 3 shows the SEM images of the AuNP1 (top row) and AuNP0 (bottom row) at different magnifications. Figures 3a and c show that the distribution of the Au NPs is homogeneous in both cases and that they are ordered in an hexagonal array. One can observe at these magnifications that the bases of the NPs are triangular and that the array is periodic. Figures 3b and d, taken at larger magnifications, exhibit the obtained structures in more detail. In particular, one can observe that these structures are not triangular but they are mainly pyramidal. Moreover, it is important to observe that, comparing AuNP1 and AuNP0, they are different in size, but they preserve almost exactly their shape, as expected.
Using the ImageJ software, it is possible to measure the size of the bases of the Au NPs. Indicating with Ab the average area of the basis and with E the average length of the edges of the triangles, we obtain, for the AuNP1, \({\text{A}_{\text{b}}^{1}}\) = 36,000 ± 3900 nm2, E1 = 289 ± 15 nm, and, for the AuNP0, \({\text{A}_{\text{b}}^{0}}\) = 7750 ± 650 nm2, E0 = 134 ± 6 nm.
An AFM study was performed to obtain more information on the height of the nanopyramids as well as on their 3D structure. The right panel of Fig. 4a shows the height of the profile of the AuNP1 nanopyramids obtained from the AFM image along the straight line drawn on the left panel. An estimated height of h1 = 44 ± 2 nm was obtained. Figures 4b and c show the 3D images of the nanopyramids, obtained with AFM and SEM respectively, at an angle of approximately 70°. This with the purpose of comparing both images and further corroborating the pyramidal geometry of these AuNP1. A similar analysis for the AFM images of AuNP0 was performed and is shown in Fig. 5. For these nanopyramids an estimated height of h0 = 47 ± 5 nm was obtained.
One can observe that in both cases the geometric structure is the same. On the one hand the AuNP1 have a larger base than the AuNP0; on the other hand the latter have a larger height, even though both NPs were obtained by evaporating the same amount (50 nm) of Au. This is due to the fact that the size of the microspheres used for the mask affects the final height of the nanopyramids. Indeed, the smaller the size of the spheres, the larger the amount of Au that penetrates.
With the data obtained regarding the size of the triangular bases and the heights of the pyramids, it is possible now to compute the lateral surface area Al. For the AuNP1, we obtain \({\text{A}_{1}^{1}}\) = 41,200 ± 3950 nm2, while for the AuNP0 we get \({\text{A}_{1}^{0}}\) = 12,270 ± 730 nm2.
These values are important because they represent the active area that contributes to the SERS effect.
NSL in combination with SSL. Here we use the Mask1 and evaporate the Au thin film on the substrates at an angle of 20°. These NPs will be labelled hereafter as “AuNP1SSL”. The results are given in Fig. 6. Figure 6a shows a 43,000 × magnification micrography exhibiting the ordered array of the bases of the nanopyramids. On the other hand, in Fig. 6b, using a larger magnification, one can observe that the bases of these Au NPs are smaller than the bases of the AuNP1 of the previous case (cf. Figure 3a). Moreover, they present a slight elongation along a fixed direction.
By performing the analysis with ImageJ, it is found that in this case two of the sides of the triangles are similar in size and they are larger than the third side. More precisely, the two larger sides have a length of approximately L = 256 ± 22 nm, while the smaller side has an approximate length of l = 183 ± 20 nm.
These sides are highlighted in Fig. 6b for one of the triangles. Moreover, the average area of the triangular bases is approximately \({\text{A}_{\text{b}}^{\text{SSL}}}\) = 21,900 ± 3500 nm2. Figure 7 shows the AFM images of the NPs and confirms the elongation of the nanopyramids. Figure 7a displays the profile of these Au NPs, together with the region of the sample where this profile was measured.
AFM images of the Au nanopyramids obtained using Mask1 and SSL: a the pattern of the triangular bases it is shown, together with the measured profile, with which we can estimate the height of the nanopyramids; b and c 3D images of the Au NPs. In b the scale bars are: 3 µm (X axis), 3 µm (Y axis), and 36 nm (Z axis)
For these AuNP1SSL an estimated height of hSSL = 17 ± 1.4 nm was obtained. Figures 7b and c show the 3D images of the nanopyramids, obtained using AFM and SEM respectively, at an angle of approximately 70°. This with the purpose of comparing both images and further corroborating the pyramidal geometry of these Au NPs.
By comparing the nanopyramids obtained by NSL (Fig. 3a) to those obtained by NSL in combination with SSL (Fig. 6a), where in both cases the same amount of Au (50 nm) has been evaporated, a large difference in size between the two structures is clearly observed, although the geometry is preserved. The most notorious effect is arguably the drastic decrease in height of the SSL nanopyramids, which is caused by the inclination of the substrate during the evaporation. Indeed, the silica particles in this situation generate a “shadow” over the Si substrate, so that the flow of the evaporated material cannot penetrate as in the case of the normal evaporation. This shadow effect is also responsible of the fact that the nanopyramids present smaller bases and an elongation along the direction of inclination of the substrate. By computing the lateral surface area, we obtain \({\text{A}_{1}^{\text{SSL}}}\) = 28,400 ± 2800 nm2. This value is the active area that contributes to the SERS effect.
3.2 Raman spectroscopy
In this section we study all the nanopyramids of different sizes that have been discussed so far as possible SERS substrates. By combining the plasmon resonance of the Au NPs with the chemical effect of the R6G we can study the amplification of the Raman signal using these substrates. To do this, first each of the substrates is functionalized with R6G at different concentrations: 10−5 M, 10−6 M and 10−7 M, respectively. Then the Raman spectra are obtained with a 532 nm laser. It is important to stress that the Raman spectra that are presented have been obtained from the same regions that have been showed in the AFM images for each case, namely Figs. 4a, 5a, and 7a.
NSL and SSL. We show here the Raman results for the nanopyramids AuNP0, AuNP1 and AuNP1SSL. The Raman signal is measured at different points on the surface of the sample, with an acquisition time of 10 s in each case. As a reference we use a 50 nm thick Au thin film, deposited over Silicon and functionalized with R6G, with precisely the same conditions as for the nanopyramids.
Figure 8 shows the Raman signal obtained using the AuNP1 and a 10−5 M R6G concentration. Clearly, the Raman signal of the R6G using the AuNP1 has a greater intensity than that obtained using the thin film. This means that these nanopyramids favor the amplification of the R6G signal, i.e., the SERS effect.
For the AuNP0 and the AuNP1SSL, the same analysis is performed. In Figs. 9, 10 and 11, the spectra for the three different types of NPs are displayed together for comparison, varying the R6G concentration, using 10−5 M, 10−6 M and 10−7 M respectively. In all cases we observe an amplification of the Raman signal of R6G due to the nanopyramids. As expected though, there is a variation in the intensity of the signal depending on the type of the Au NPs. This is due to the different values of the active area for each case, that is, smaller nanopyramids tend to have a smaller lateral surface area with which the R6G molecules can couple, and therefore one gets a decrease in the Raman-SERS signal. Nevertheless, even with the smallest nanopyramids, a good amplification is observed, in comparison with the Au thin film, for which the signal is barely detectable.
Figure 12 shows the variation of the Raman signal intensity as a function of the R6G concentration for each type of Au NPs. As a reference, we used the intensity of the first peak (at 613 cm−1). The intensity of the signal depends on both the active area of the Au NPs and the R6G concentration. The first result is that at each concentration the AuNP1 exhibits the largest intensity. This is expected from the previous analysis, as the AuNP1 have the greatest active area among these Au NPs. We also notice that in each case the intensity is an increasing function of the concentration. Also expected is the fact that by decreasing the dye concentration, the active area plays a less important role. This is because there are less molecules to be distributed on the NPs surface, independently of the active area. This phenomenon can be corroborated in Fig. 12 by the fact that all the curves tend to merge at the smallest concentration, while at higher concentrations they are well separated. We also expect and presume that each of the Au NPs should display a “saturation effect” at higher concentrations. By this we mean that if we continue to increase the concentration of the R6G, at some point the number of molecules will overcome the available active area in each case, and therefore one should observe that the curve should stop increasing. A detailed study of this behavior will be the subject of a forthcoming work.
Enhancement Factor. In order to quantify the amplification of the Raman-SERS signal in each case, we compute the Enhancement Factor (EF), defined by Le Ru et al. in [33] as
Where Ibulk is the intensity obtained with the Au film, Isurf is the intensity obtained with the NPs, Nbulk is the number of molecules measured on the Au film and Nsurf is the number of molecules measured on the Au NPs. For all cases, the 532 nm laser is used. Table 1 shows the results of the corresponding EF. In all cases the EF is of the order of 105–106, as it can also be foreseen qualitatively from the Raman spectra. This means that all the Au NPs considered here present a large amplification of the Raman signal, even at very low concentration of the R6G. We conclude that these Au NPs are a good option in general as detectors of organic molecules, such as dyes.
4 Conclusions
Spherical silica particles of 650 and 465 nm of diameter were fabricated using the Stöber method. They were then used to obtain lithographic masks using a spin coating system, producing a compact monolayer array of the spheres over Si substrates. After evaporating a 50 nm thick Au thin film over the lithographic masks and removing the masks, we obtained pyramidal Au NPs. Thorough SEM and AFM analysis were performed, and the results corroborated that indeed different sizes of nanopyramids were fabricated, depending on the silica particle diameter and the use of either NSL or SSL. For the Raman-SERS analysis, we employed Rhodamine 6G at a concentration of 10−5 M, 10−6 M and 10−7 M. An increment in the Raman signal of the characteristic peaks of the Rhodamine was observed for nanostructured substrates prepared with NSL and SSL in comparison with the signal obtained using the bare Au film, which indicates that all the Au nanopyramids, regardless of their sizes, are good candidates as SERS substrates for the detection of dyes at low concentrations.
For future work, we expect to extend the present analysis in order to compare further the behavior of the substrates obtained by SSL and NSL respectively when the concentration of the dye is varied and for different dyes (e.g. Methylene blue [27] and Methyl orange [34]). In each case, we will optimize the free parameter (tilt angle) during the fabrication of the SSL substrates and then investigate the detection limits of the Au NPs. Our main interest is to develop applications to determine low concentration dye contaminants in water, and their removal by photocatalysis.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
G.L. Hornyak, J. Dutta, H.F. Tibbals, A. Rao, Introduction to Nanoscience (CRC Press, 2008)
S. Chupradit, M. Kavitha, W. Suksatan, M.J. Ansari, Z.I. Al Mashhadani, M.M. Kadhim, E. Kianfar, Morphological control: properties and applications of metal nanostructures. Adv. Mater. Sci. Eng. 2022, 1–15 (2022)
U.Y. Qazi, R. Javaid, A review on metal nanostructures: preparation methods and their potential applications. Adv. Nanoparticles 5(01), 27 (2016)
G.L. Hornyak, J.J. Moore, H.F. Tibbals, J. Dutta, Fundamentals of Nanotechnology (CRC Press, 2018)
S. Lindsay, Introduction to Nanoscience (Oxford University Press, 2010)
Dupas, C., Lahmani, M. (Eds.). (2007). Nanoscience: Nanotechnologies and Nanophysics. Springer Science & Business Media.
H.W. Deckman, J.H. Dunsmuir, Natural Lithography. Appl. Phys. Lett. 41(4), 377–379 (1982)
J.C. Hulteen, R.P. Van Duyne, Nanosphere lithography: a materials general fabrication process for periodic particle array surfaces. J. Vac. Sci. Technol. A 13(3), 1553–1558 (1995)
Burkhardt, C., Fuchsberger, K., Nisch, W., Stelzle, M. (2010). Micro-and Nanopatterning of Surfaces Employing Self Assembly of Nanoparticles and Its Application in Biotechnology and Biomedical Engineering. In Lithography. IntechOpen.
C.L. Haynes, A.D. McFarland, M.T. Smith, J.C. Hulteen, R.P. Van Duyne, Angle-resolved nanosphere lithography: manipulation of nanoparticle size, shape, and interparticle spacing. J. Phys. Chem. B 106(8), 1898–1902 (2002)
B. Ai, Y. Zhao, Glancing angle deposition meets colloidal lithography: a new evolution in the design of nanostructures. Nanophotonics 8(1), 1–26 (2018)
B. Myint, D.S.F. Yap, V. Ng, Stepwise nanosphere lithography: an alternate way of fabricating nanostructures. Nano Exp. 1(2), 020029 (2020)
Y. Wang, D. Ye, F. Zhao, Z. Zhang, Y. Zhao, Structure and optical property prediction of 2D plasmonic photonic crystals fabricated by shadow sphere lithography. ACS Appl. Nano Mater. 5(12), 17879–17890 (2022)
K. Kim, K.J. Lee, N.R. Jo, E.J. Jo, Y.B. Shin, M.G. Kim, Wafer-scale LSPR substrate: oblique deposition of gold on a patterned sapphire substrate. Biosensors 12(3), 158 (2022)
S. Kumar, S.K. Gahlaut, J.P. Singh, Sculptured thin films: over- coming the limitations of surface-enhanced Raman scattering substrates. Appl. Surface Sci. Adv. 12, 100322 (2022)
K. Namura, S. Hanai, S. Kondo, S. Kumar, M. Suzuki, GoldMicropetals self-assembled by shadow-sphere lithography for optofluidic con-trol. Adv. Mater. Interfaces 9(18), 2200200 (2022)
S. Yadav, S. Senapati, S. Kumar, S.K. Gahlaut, J.P. Singh, GLAD based advanced nanostructures for diversified biosensing applications: recent progress. Biosensors 12(12), 1115 (2022)
J.H. Han, D. Kim, J. Kim, G. Kim, P. Fischer, H.H. Jeong, Plasmonic nanostructure engineering with shadow growth. Adv. Mater. 35(34), 2107917 (2023)
Y. Zhao, A. Kumar, Y. Yang, Unveiling practical considerations for reliable and standardized SERS measurements: lessons from a comprehensive review of oblique angle deposition-fabricated silver nanorod array substrates. Chem. Soc. Rev. 53, 1004 (2024)
A.A. Osipov, A.E. Gagaeva, A.B. Speshilova, E.V. Endiiarova, P.G. Bespalova, A.A. Osipov, S.E. Alexandrov, Development of controlled nanosphere lithography technology. Sci. Rep. 13(1), 3350 (2023)
M. Domonkos, A. Kromka, Nanosphere lithography-based fabrication of spherical nanostructures and verification of their hexagonal symmetries by image analysis. Symmetry 14(12), 2642 (2022)
M. Sackmann, A. Materny, Surface enhanced Raman scattering (SERS)–a quantitative analytical tool? J. Raman Spectrosc. 37(1–3), 305–310 (2006)
I.E. Pavel, K.S. Alnajjar, J.L. Monahan, A. Stahler, N.E. Hunter, K.M. Weaver, D.A. Dolson, Estimating the analytical and surface enhancement factors in Surface-Enhanced Raman Scattering (SERS): a novel physical chem- istry and nanotechnology laboratory experiment. J. Chem. Educ. 89(2), 286–290 (2012)
K. Zhang, T. Zeng, X. Tan, W. Wu, Y. Tang, H. Zhang, A facile surface-enhanced Raman scattering (SERS) detection of rhodamine 6G and crystal violet using Au nanoparticle substrates. Appl. Surf. Sci. 347, 569–573 (2015)
F. Tian, F. Bonnier, A. Casey, A.E. Shanahan, H.J. Byrne, Sur- face enhanced Raman scattering with gold nanoparticles: effect of particle shape. Anal. Methods 6(22), 9116–9123 (2014)
P. Zhang, Y. Jin, J. Fang, Triangular Au nanoparticle arrays based on flexible materials as temperature-sensitive SERS substrates. Opt. Mater. 146, 114556 (2023)
M. Zannotti, A. Rossi, R. Giovannetti, SERS activity of silver nanosphere, triangular nanoplates, hexagonal nanoplates and quasi-spherical nanoparticles: effect of shape and morphology. Coatings 10(3), 288 (2020)
W. Stober, A. Fink, E. Bohn, Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26(1), 62–69 (1968)
C.J. Brinker, G.W. Scherer, Sol-gel science: the physics and chemistry of sol-gel processing (Academic Press, 2013)
R. Lu, A. Konzelmann, F. Xu, Y. Gong, J. Liu, Q. Liu, M. Xin, R. Hui, J.Z. Wu, High sensitivity surface enhanced Raman spectroscopy of R6G on in situ fabricated Au nanoparticle/graphene plasmonic substrates. Carbon 86, 78–85 (2015)
C.A. Schneider, W.S. Rasband, K.W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9(7), 671–675 (2012)
C. Salinas et al., Fabrication and characterization of Surface-Enhanced Raman scattering substrates with ordered arrays of gold nanopyramids by means of nanosphere lithography. Mater. Exp. 9(2), 141–149 (2019)
L. Ru, G. et al., Surface Enhanced Raman scattering enhancement factors: a comprehensive study. J. Phys. Chem. C 111(37), 13794–13803 (2007)
M.F. Hanafi, N. Sapawe, A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today: Proc. 31, A141–A150 (2020)
Acknowledgements
The authors are grateful to S. Tehuacanero and R. Hernández (IF, UNAM) for helping with the SEM, to M. Avendaño and G. Ramírez (IE-SEES, CINVESTAV-IPN) and C. Zorrilla (IF, UNAM) for helping with the Raman measurements and to J. G. Morales (IF, UNAM) for the thin film evaporation. Cecilia Salinas-Fuentes acknowledges support from CONAHCYT, program Estancias Posdoctorales por México. This work was supported by PAPIIT-UNAM under project IN-110123.
Funding
Cecilia Salinas-Fuentes acknowledges support from CONAHCYT, program Estancias Posdoctorales por México. This work was supported by PAPIIT-UNAM under project IN-110123.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Cecilia Salinas-Fuentes and Angélica Hernández-Zanabria. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salinas-Fuentes, C., Hernández-Zanabria, A., Cheang-Wong, JC. et al. Modified nanosphere lithography: application to the fabrication of ordered arrays of gold nanopyramids. J Mater Sci: Mater Electron 35, 1680 (2024). https://doi.org/10.1007/s10854-024-13289-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-13289-7