Abstract
A graphene-based Sm-doped Co-ferrite CoSmFe-G nanosample was created using the citrate auto-combustion method. It acts as an antibacterial agent and heavy metal remover. The sample’s physical properties were analyzed using various techniques including X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, scanning electron microscopy (FE-SEM), zeta potential, and vibrating sample magnetometer (VSM) measurements. The XRD results confirmed the presence of a single-phase cubic spinel structure. The experimental results indicated that doping with graphene and samarium significantly altered the magnetic properties of CoSmFe-G nanoparticles, reducing the saturation magnetization (MS) values to 35.759 emu/g. The sample showed antibacterial properties against both gram-positive and gram-negative bacteria, with inhibition zones measuring 12–14.5 mm in diameter. Additionally, the sample exhibited efficient (Pb2+) adsorption, with a maximum removal capacity of 99.8% and a multilayer adsorption mechanism indicated by the pseudo-second-order isotherm and Freundlich model matching. The study explores the binding interactions for lead removal and highlights the potential of using graphene-based Sm-doped Co-ferrite (CoSmFe-G) nano samples as a bio-adsorbent and antibacterial agent. This could lead to practical and efficient solutions for water disinfection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites are significant in numerous industrial uses because of their superior magnetic, biocompatible, as well as adsorption capabilities, they have characteristics of both magnetic materials and insulators. Transitional metal oxides, such as cobalt oxides, are often used in heterogeneous catalysis. They play a crucial role in the development of rechargeable batteries, CO sensors, and in air pollution reduction [1, 2].
The properties of spinel type ferrites are influenced by preparation method, microstructure, chemical composition, and particle size and shape. Adding other cations like Co2+ can enhance ferrite characteristics, expanding their applications. Adjusting the type as well as the amount of substituted cation can customize co-ferrite’s properties. Mixed Mn–Co ferrites have improved dielectric and magnetic properties, suitable for applications involving high magnetostriction, magneto-mechanical, magneto-optical, embedded stress sensing, and non-contact torque sensing [3].
Many applications, including those in the electronic industry, permanent magnets, microwave devices, microwave absorbers, oscillators, phase shifters, filters, magnetic ferrofluids, magnetic refrigeration, magnetic storage discs, magnetic separation, switching, core, recording heads, magnetic shielding, and high-frequency devices, have made use of magnetic spinel nanoferrites. On the other hand, the soft and hard magnetic behavior of various applications determines their classification. It has been studied whether superexchange interactions can lead to an improvement in the structural and magnetic characteristics of spinel nanoferrites by substituting metal ions. There are also effects on these qualities [4]. The creation of fine-grained microstructures through chemical means often results in poor yields and requires lengthy reaction times and post-annealing steps. To prevent particle agglomeration and control particle shape and size, different surfactants may be added at various stages of the synthesis The creation of transition metal doped ferrites involves several techniques such as sol–gel, combustion, powder ceramic, thermal breakdown, electrospinning, solid-state reactions, co-precipitation, microemulsion, laser pyrolysis, and the microwave-hydrothermal method. Among these, the sol–gel method is the most straightforward and cost-effective, as it requires a low processing temperature. Nitrate salts are preferred precursors for synthesizing spinel ferrites because they are considered water-soluble low-temperature oxidizing agents [5].
The sol–gel process is commonly used in the production of nanoscale ferrites due to its cost-effectiveness, adaptability, ease of use, and ability to control surface, physical–chemical, and structural characteristics. However, this method has drawbacks such as the presence of secondary crystalline phases at high annealing temperatures, amorphous phases at low annealing temperatures, and irregular nanoparticle shapes due to gas evolution during reactant breakdown [6]. Another popular method for synthesis nanoparticles of desired sizes and shapes is co-precipitation. On the other hand, the solid-state reaction produces nanoparticles with higher magnetostriction at lower magnetic fields. Combustion synthesis is also favored for producing high-purity samples of various sizes and shapes due to its benefits, which include simplicity, affordability, quick reaction time, and low energy consumption [5]. The optical, electrical, magnetic, as well as dielectric properties of transition metal spinel ferrites (MFe2O4, M = Co, Cu, Mn, Ni, Zn) as well as their chemical stability make them intriguing materials. The synthesis process, concentration, kind, composition, and placement of the cations all affect the structure and characteristics of ferrites. Furthermore, compared to bulk size particles, the nanoscaled ferrite particles exhibited superior optical, electrical, magnetic, and catalytic behavior [7]. Additive substitutions, calcination processes, and synthesis techniques can all affect the ferrite structure and magnetic characteristics. Their magnetic interaction and cation distribution in the two sublattices, namely the tetrahedral (A) along octahedral (B) sites, determine their structural, electrical, as well as magnetic properties. The ferrite magnetic properties are largely dependent on the size and shape of the particles. There is a critical particle size less than which a single-domain structure can transition to a multidomain structure in nanoscale magnetic materials. Within single-domain systems, magnetizing anisotropy controls the spin proper alignment along the magnetization axis below the so-called blocking temperature [8].
These lattice interactions at tetrahedral and octahedral sites make them more significant, and this could be beneficial for the tuneable features of nanoferrites, enhanced remanence, low coercivity (Hc), high resistivity, chemical stability, big saturation magnetization (Ms), and favorable electromagnetic compatibility [9]. In the inverse spinel arrangement of cobalt ferrite, the tetrahedral (A) sites have been occupied with Fe3+ ions (rFe3+ = 0.49 Å), while the octahedral (B) sites have been shared equally between divalent Co2+ (rCo2+ = 0.745 Å) as well as Fe3+ [10]. It has a Curie temperature (TC) of about 520 °C and as such is ferrimagnetic. It also exhibits a rather strong magnetic hysteresis, that sets it apart from the other spinel ferrites [11]. Reduced magnetic hyperfine field and Curie temperature indicate the potential for manipulating magnetic coupling with the substitution of other elements in CoFe2O4 ferrite [12]. The main challenge facing today’s world, among the many universal problems, is finding clean, pure water [13]. Many hazardous and poisonous effluents produced by many companies are thrown into water sources, causing severe pollution and contamination of the water. Researchers from around the world are attempting to create new photocatalytic materials with improved oxidative degradation properties to eliminate fabric organic dye effluents which cause significant water pollution. Another potential hazard to human health and the source of worry for millions of people worldwide has been the fast rise in infectious diseases caused by bacteria [14]. However, antibacterial substances effectively guard against these fatal infectious infections. However, the sudden usage of conventional antibiotics has paved the way for the emergence of numerous drug-resistant bacteria, which cause irreversible diseases. The newly created adsorption and antibacterial materials, such as CaFe2O4, ZnFe2O4, and WO3, showed good results because of their magnetic properties [13]. Ferrites’ adsorption and antibacterial properties are constrained, however, by creating composites of ferrites doped with rare earth and graphene, this issue can be solved as ferrites’ capacity for adsorption of heavy metals and antibacterial is improved. According to reports, the substitution of rare earth ions caused structural distortion and considerable alterations in electrical as well as magnetic properties [15, 16]. This can be explained by the fact that for rare earth, the unpaired 4f e’s and significant spin–orbit angular momentum coupling have been identified as properties of rare earth (RE) ions. The Fe–Fe interactions (3d e’s spin coupling) affect both the magnetic and electrical attributes of ferrites [15]. Rare earth ions (R) have been integrated into the spinel lattice, which causes the R-Fe interaction (3d–4f coupling) which improves the ferrites’ electrical as well as magnetic, magneto-optical, and physical properties. Magneto crystalline anisotropy for spinel ferrites is likewise determined by this phenomenon. Spinel ferrites can now use RE ions as substitutes to enhance their electromagnetic characteristics [16]. While the addition of graphene G has essential effects because of its exceptional electrical, mechanical, thermal, and chemical properties, graphene has recently received a lot of interest. There are numerous applications for the combination of magnetic nanostructure with graphene, including catalysis, biomedical fields, and the removal of pollutants from wastewater. As a result, research interest in recent years has focused on the investigation of the exceptional magnetic, optical, and electrical attributes of rare earth doped spinel ferrites. Numerous researchers have been interested in rare earth doped ferrites recently due to their peculiar magnetic, optical, as well as electrical properties. The utilization of cobalt ferrite CoFe2O4 as an environmentally sensitive material for the removal of heavy metals and antibacterial material was discussed before [17]. According to a review of the literature, CoFe2O4 is crucial in environmental applications [18, 19]. Also, large mechanical strength, heat stability, and high magnetization are among its main characteristics [20]. The rare earth lanthanides have a high resistance and are effective electrical insulators. They are frequently employed to change the ferrites’ structural, magnetic, as well as electrical properties [21]. They are said to provide high DC resistivity, extremely low electrical permittivity, and minimal dielectric loss when substituted. Numerous studies have looked at the effects of substituting rare earth ions including La, Sm, Gd, Dy, Yb, Er, Tb, and Y for Fe3+ ions. On the other hand, the addition of graphene demonstrates how it directly affects the nanoferrite’s crystalline structure and magnetic characteristics. Dual adding is carried out to see a further improvement in the magnetic characteristics. To examine the structural, electrical, and magnetic properties, the first attempt has been undertaken to synthesize G added to Sm-doped cobalt ferrites with formula (CoSm0.025Fe1.975O4)0.9G0.1. Additionally, this work offers the benefit of describing the biosafety of antibacterial compounds based on graphene. It is hoped that this study will stimulate new ideas and offer important current insight for the continued creation of safe and effective antibacterial compounds based on graphene. Furthermore, despite the exceptional antibacterial and heavy metal removal, and efficacious qualities of these graphene-based rare earth nanoparticles, there is still cause for worry regarding their potential clinical uses. These elements’ desired qualities may also be equally linked to unanticipated as well as biohazardous toxicities. Consequently, to emphasize the significance of their clinical translation, the prospects and difficulties for the future will also be included.
Spinel nanoferrites are straightforward and inexpensive to fabricate, which makes them promising for a wide range of uses. Improvements in characteristics are mostly due to super exchange interactions between spinel nanoferrites at the lattice locations [9].
The microwave method, glass crystallization method, co-precipitation, sol–gel auto combustion, and mechanical alloying are some of the various synthesis procedures [22].
Due to its superior end characteristics, the improved homogeneity, shape, and results of the prepared nanoferrites, fine surface morphology, and low-temperature need for ferrite in synthesis, sol–gel auto combustion has received a lot of interest lately. To adjust the structural, electrical, and magnetic properties of spinel-based structures, metal cation substitution will be useful. For industrial and technological applications, ferrites with reduced magnetic losses and eddy currents are more affordable [9].
Graphene-based nano spinel ferrites have a low electronic bandgap, an extensive specific surface area, and excellent chemical stability, which may make them better than other materials. Although the graphene prevents the toxic nanoparticles from leaching, the ferrites that form on the graphene nanosheets may inhibit agglomeration. Additionally, graphene-based nanocomposites have the following characteristics: large area of surface, elevated stability, Chemical composition that can be adjusted, enhanced electrical characteristics, and enhanced conductivity. The synthesis of samarium-graphene-magnetic nanoferrite has not been studied extensively. However, this work successfully produced a CoSmFe-G nanocomposite with adjustable magnetic characteristics using the citrate auto-combustion method. A graphene-based CoSmFe-G nanosample shows potential as an antibacterial agent and heavy metal remover. Various techniques confirmed its physical properties, including the presence of a single-phase cubic spinel structure. This study is significant as it is the first attempt to use CoSmFe-G as biomaterials for adsorption and photoactivated antibacterial applications. The antibacterial properties of CoSmFe-G against both Gram-positive and Gram-negative bacteria, as well as its impact on the removal of lead from wastewater, are also investigated. In addition to its biological focus, the study also aimed to examine the shape, magnetic properties, and microstructural behavior of the sample.
2 Experimental
2.1 Materials and preparations
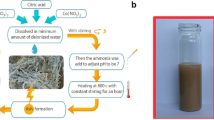
The used chemicals were purchased from Alfa Aesar with a purity of 99.9%. Graphene was prepared using a modified Hummer method [23]. CoSmFe-G was prepared by mixing stoichiometric ratios of cobalt, samarium, iron nitrates, and graphene with citric acid by the citrate auto-combustion technique. By using 0.026 M of iron nitrate, 0.013 M of cobalt nitrate, 3.33 × 10–4 M of samarium nitrate and 0.04 M of citric acid. Cations/citric acid molar ratio was 1:1. Citric acid is used as a fuel to reinforce the mixing homogeneity. 0.09 M Graphene was added by weight percent 10%. According to reported literature in Table 1 the sintering temperature was chosen. The synthesis flow chart is shown below in Fig. 1.
2.2 Characterizations of the investigated sample
Using CuKa radiation (k = 0.15405 nm) X-ray diffractometer (XRD), the structure as well as crystallite sizes have been investigated. Fourier-transformed infrared FT-IR spectra were obtained using an FT-IR spectrometer (PerkinElmer 2000) in the wavelength range of 4000 cm−1 to 390 cm−1. Raman analysis was performed by Confocal Raman Microscope (WiTec, 300R alpha, made in Germany) laser source 532 nm. For surface study and morphology field emission scanning electron microscopy FE-SEM was used. The magnetic properties were studied by using VSM. The prepared specimen’s zeta potential has been determined by a zeta seizer nano series (Nano ZS), Malvern, UK, with a size from 0.6 to 6000 nm as well as a zeta potential spectrum of (− 200 to 200 mV).
2.3 Antibacterial activity
Gram-negative bacteria (E. coli, P. aeruginsa, and S. typhimurium) and Gram-positive bacteria (S. aureus, B. cereus, and S. faecalis) were acquired under the American Type Culture Collection (ATCC). Tryptone soy broth (TSB) (Oxoid), as well as trypticase soy agar (TSA) (Difco), were the media utilized for bacterial growth. (CoSm0.025Fe1.975O4)0.9G0.1 ferrite powders were used at a starting concentration of 5 mg/ml in standard agar plate experiments. The samples were incubated with bacteria for a full day at 37 °C. The inhibitory zones were subsequently measured. The control sample did not include any of (CoSm0.025Fe1.975O4)0.9G0.1 but was injected with bacteria.
2.4 Adsorption study of (CoSm0.025Fe1.975O4)0.9G0.1
Employing a batch sorption test, the adsorption effectiveness of the investigated sample was examined. Initially, 2000 mg/l of Pb (NO3)2 was dispersed in deionized water while being violently shaken using an electric shaker (ORBITAL SHAKER SO1) to create a standard solution containing heavy metal ions. By adjusting the experimental conditions, including solution pH and contact time, the optimum adsorption of (Pb2+) was observed. The pH level of the solution was subsequently modified at various levels (2–9) after 0.02 g of the sample that had been prepared had been dissolved in 20 ml of the standard solutions. A syringe filter was used to collect the transparent solution shortly after an hour, and then ICP spectroscopy was used to determine the lead ion concentration. The ideal contact time was adjusted when the best pH value was identified by combining 0.1 g of the prepared sample with 100 ml of the standard solution to keep the pH at the desired level (Pb2+) content was determined at various intervals (10–60 min). The equation was used to compute the sample’s removal efficiency [30]
where; C0: is the beginning concentration of metallic solution (ppm) and Cf is the final concentration of heavy metallic solution (ppm).
3 Results and discussion
3.1 Structure properties
XRD analysis is used to examine the cubic crystalline structure of synthetic (CoSm0.025Fe1.975O4)0.9G0.1 NPs. The corresponding Miller indices associated with the diffracted peaks were appropriately matched to JCPDS card numbers 00-022-1086 and 00-026-1076 which are related to CoFe2O4 and Graphene, respectively. The high-intensity peak related to (311), which is seen in Fig. 2, confirmed the produced NPs’ single-phase cubic composition [31]. Figure 2 clearly shows the addition peak at 26.61 (002), which corresponds to the graphene, demonstrating the existence of CoSm0.025Fe1.975O4 along with graphene in the sample [32]. No more peaks or contaminants can be seen in the XRD spectrum of the investigated sample proving that Sm has been thoroughly doped within the cobalt ferrite [33]. The Scherrer’s equation and W–H plot [34, 35] were used to determine the average crystallite size as well as lattice properties of the synthesized co-doped cobalt ferrite NPs:
Throughout the equation, D stands for crystallite size, d for d spacing, a for lattice parameter, and hkl for the produced co-doped synthetic specimens’ lattice planes.
The dopant sample has a D of 29 nm, whereas the D of the CoFe2O4 sample was determined previously in [28] to be 31.37 nm. This decrease can be attributed to many issues. The most crucial of them are electronic configuration, valences, and the difference in ionic radii between rare earth ions and the Fe3+ ion. Moreover, the bond energy of RE3+–O2− is greater compared to Fe3+–O2−, the extra amount of energy is required to incorporate RE3+ ions in cobalt lattice to form RE3+–O2− [36]. Hence extra energy that is required hinders the crystallization process and growth of grains in the doped samples [37]. Therefore, a reduction in crystalline nature with smaller crystallite size is detected. On the other side, there is a solubility limit for the replacement of Fe3+ ions by RE ions in the cobalt ferrite lattice. In the present study, the disappearance of secondary phases indicates a complete solubility of rare earth ions in the ferrite matrix. Thus because the ionic radii of Sm3+ ions (0.958 A) are bigger compared to those of Fe3+ ions (0.645 A), the size of crystallites has decreased. The M–O separation of the produced samples as well as the greater bonding among the Sm3+–O ions compared to the Fe3+–O ions are further causes for the reduction in crystallite size [38]. Our calculations show that Sm3+ ions prefer to be in the octahedral position because their ionic radius is greater than that of Co2+ and Fe3+ present there. As a result of the increased space within the interstitial octahedral location compared to the tetrahedral location, Sm3+ ions prefer to reside there [39].
The produced NPs’ cell volume, dislocation, and micro-strain have been calculated using the formulae found in the literature [17, 34, 35, 40,41,42] and are shown in Table 2. The various changes in the lattice parameters and the expansion of the unit cell volume resulted from doping which creates stress and expansion in the spinel lattice [32, 43, 44].
Using the Williamson–Hall method, the lattice strain (\(\varepsilon )\) in the sample is calculated as shown in Fig. 3. The Williamson–Hall equation [45] is expressed as
since the term \(4\varepsilon \text{sin}\theta\) is called the strain effect. The graphs are linearly fitted. The lattice strain is the slope of the straight line. Because the stress field from grain boundaries can force atoms in the crystallites to deviate from their ideal positions, this can account for the decrease in micro-strain. This effect, which is mostly centered close to the grain boundaries, modifies the lattice parameters in addition to producing a local lattice strain [46].
The standard deviation in statistics measures how much a random variable is expected to vary from its mean. The calculated standard deviation for the crystallite size was found to be 0.70. The values are relatively close to the mean because the standard deviation of the dataset is 0.7. Specifically, approximately 68% of the data points within a normal distribution fell within one standard deviation of the mean. Therefore, given a standard deviation of 0.7, about 68% of the data points lie within 0.7 units of the mean. The standard deviation decreases as the data points get closer to the mean and increases as the data points become more dispersed. A dataset with a standard deviation of 0.7 indicates that the data points are more likely to be close to the mean compared to a dataset with a higher standard deviation, regardless of the independent variable.
Table 3 summarizes the crystallite size comparison between our work and that found in the literature. It was discovered that undoped spinel ferrites have a higher crystallite size value than doped ferrites. The doping regulate and retard the growth of the crystallite size, according to the results. Our sample has the smallest crystallite size, as shown in Table 2, which has a significant effect on environmental applications because smaller crystallite sizes can result in higher antibacterial activity. Bacteriophagous metal particles exhibit bactericidal activity against microorganisms. Because smaller particles possess a greater surface area as well as sites for adsorption accessible, they are capable of elimination heavy metals with higher removal efficiencies. This is due to the fact that active sites and raised specific surface area work better together in smaller particle sizes.
FT-IR was used to examine the functional groups, and metallic ion stretching, along with spectroscopic analyses of the Sm-doped cobalt ferrite based on graphene NPs. Figure 4 displays the FT-IR spectrum of these NPs in the 390 cm−1 to 4000 cm−1 range. Throughout the 400 cm–1 to 600 cm–1 wavenumber region of the FTIR spectrum, two distinct bands were seen as a result of the vibrations by the metal cations found in the synthesized spinel ferrite arrangements, such bands were ascribed to spinel ferrite. The cation distribution in the tetrahedral as well as the O within the synthesized CoSmFe-G nanosample was represented by the bands that were detected (400 cm−1 to 600 cm−1). The metal ion stretching location at the octahedral sites was described by a low absorption band around 600 cm−1, while high-frequency bands were represented by a band about 400 cm−1 [52]. The Carboxyl group is responsible for the absorption bands in the ranges of 2360 cm−1 [53]. The O–H vibration has a wide peak at 3440 cm−1, whereas the C–H stretch has a band at 2846 cm−1 [54,55,56,57,58,59]. A peak at 1730 cm−1 is credited with the skeletal vibration in graphene (C–C) [60].
An excellent, accurate, and quick technique for characterizing graphene after chemical treatment is Raman spectroscopy. For graphene-based structures, the G and 2D bands are the two main bands that define the spectra’s rather simple structure. When imperfections in the carbon lattice are present, a third band, known as the D-band, which is referred to as the defect band or the disorder band can occasionally be observed in graphene [61]. Room-temperature Raman analysis was carried out as illustrated in Fig. 5. The prominent peak detected at about 1590 cm−1 was named G-bands and was linked to graphene’s sp2 structure confirming the presence of graphene. The position of the G-band is highly sensitive to the number of layers in the sample. The peak detected at about 1363 cm−1 was linked to the D-band and was determined to be caused by defects in the structure of the graphene lattice. For graphene, the 2D band is consistently a strong band. It is observed that the 2D band has a single symmetric peak confirming a single-layered graphene structure [62]. Additional peaks can be seen in the Raman spectra in the range 100–1000 cm−1, which can be attributed to the stretching vibration of Cobalt samarium spinel ferrite [63, 64].
3.2 Microstructural analysis
FE-SEM was used to examine the graphene-based Co-Sm, CoSmFe-G nanosample morphology after they had been prepared. FE-SEM findings are shown in Fig. 6. The findings showed that the grains are amorphous nanoparticles. The samples’ high magnetic properties can be accused of the agglomeration that occurred during the formation of microstructure. The behavior seen in other rare earth doped Co-ferrite has a similar pattern [11]. Based on our previous work and other literature [65, 66], the outcomes demonstrated that for CoFe2O4 nanocrystals calcined at 500 °C, the microstructure of the nanoparticles was virtually regular in shape and scattered evenly. Spinel began to deform with the addition of Sm as well as graphene and the resulting powders were discovered in two layers, one of which was determined to have extremely fine powder coating the spinel network. Due to their contact with the graphene surface, Sm-doped CoFe2O4 nanoparticles were integrated into the graphene, creating cluster-like structures of 90 nm. This micrograph demonstrates how magnetic nanoparticles and graphene were successfully combined to create the magnetic nanocomposite. The ImageJ software was used to determine the co-doped spinel ferrite NPs’ average particle size [30, 67]. The prepared NPs’ average particle size was 90 nm and ranged from 20 to 160 nm. Additionally, EDX analysis verified the graphene-based for CoSmFe-G elemental makeup. Co, Fe, C, and O atoms are seen in Fig. 7 together with no other impure elements.
3.3 Magnetic properties
The composition, grain size, as well as cation distribution among the octahedral and tetrahedral positions in the spinel structure related to ferrites all have a significant impact on their magnetic characteristics. As can be observed in Fig. 8, the sample displays standard soft ferrimagnetism behavior, since it’s nanoparticle size, its magnetizations reach saturation under an external field in the range of 15 kOe. Graphene-based Sm-doped CoFe2O4 powder sample’s M–H loop was measured at room temperature by a vibrating sample magnetometer (VSM), and the outcomes are shown in Fig. 8. The saturation magnetization (MS) and other magnetic properties such as residual magnetization (Mr) as well as coercive field (HC). Based on the magnetization curves were derived. (MS), (Mr), and (HC) values from VSM Fig. 8 were 35.759 emu/g, 15.274 emu/g, and 1685.2 Oe correspondingly, while the corresponding values for pure Co-ferrite as computed and analyzed in prior work [28] were 66.847 emu/g, 31.114 emu/g, and 1641 Oe. The following equation [60] is used to compute the magnetic moment measured in Bohr magnetron (μB), in which M denotes the molecular weight of a certain ferrite composition and (MS) indicates saturation magnetization (emu/g).
One could calculate the anisotropy constant, K (erg/g), as follows:
The degree of magnetic anisotropy is directly correlated with coercivity; that is, the higher the anisotropy, the more resistant the dipoles are to having their magnetically coupled arrangement destroyed by an external field applied in the opposite direction. As anticipated, Graphene-based Sm-doped CoFe2O4 powder sample’s K value, measuring 62.77 × 103 erg/g. The dependence of magnetic properties upon a preferred crystallographic direction is known as magnetic anisotropy. Deflecting the magnetic moment within a single crystal going from the easy toward the hard direction regarding magnetization requires a certain amount of energy.
Multiple factors can have an impact on these findings: (a) super exchange among A-site and B-site cations and RE3+ replacing Fe3+ in the spinel lattice [68, 69]. RE3+–Fe3+ (3d–4f coupling) and RE3+–RE3+ (4f–5d–4f coupling) interactions can be caused by traces of RE3+ within the lattice. (b) As the defects in crystal structure increased by doping, it collected on the grain boundaries and blocked the magnetic response associated with the samples; (c) As RE3+ ions have a smaller effective magnetic moment (1.38 μB) than Fe3+ ions (5.9 μB) [70], this might be the cause of the reduced MS.
In other words, undoped cobalt ferrite has a larger magnetic moment, but when Sm is added, the value of the magnetic moment continues to decline [71]. The deterioration of AB-exchange interactions, where A and B correspond to the element supporting the general spinal formula provided as AB2O4, can also be attributed to the decline in saturation, magnetization, and remanence. Rare earth (RE) like Sm3+, in which the 4f electrons are often highly localized within the material and give rise to magnetic electrons. This is comprehensible considering the orbital screen effect. As a result, the 5d as well as 6s e’s shield the 4f e’s from the crystal field. As a result, it appears that the impact of graphene-based Sm atoms in cobalt ferrite materials is comparable to that of substituting non-magnetic atoms in the octahedral Fe positions of the spinel lattices. Because the quantity of magnetic ions occupying the tetrahedral as well as octahedral sites determines the net magnetic moments within the ferromagnetic ferrite. The squareness ratio value (Mr/Ms) is about 0.5, confirming the single-domain magnetic structure of CoSmFe-G nanoferrites. As a result, in the domain movement increases with increasing grain size. Lessening the grain size limits domain mobility, in addition to the shielding phenomenon, in which a non-magnetic phase, like graphene, shields the ferrimagnetic grains from the effects of the applied field. Increasing the HC in the substituted compounds from 1641 Oe [28] to 1685.2 Oe. Additionally, the exchange bias (HEB) of the applied field might be represented as [72].
where H (−) and H (+) are the magnetization’s intercepts with the −ve and +ve on the field axis, respectively. HEB values for undoped and doped ferrites are 2 [73] and 0.1 Oe, respectively; however, this drop in value was caused by the interaction of the ferrimagnetic components with the non-magnetic grains in the sample [17].
The magnetization derivative, dM/dH, is related to the applied magnetic field for the CoSmFe-G nanosample.is shown in Fig. 9. The anisotropy constants (K), anisotropy field (Ha), and the switching field distribution (SFD) of the graphene-based-co-doped NPs were calculated using the following equations [73, 74]:
The coercivity has a significant relationship with the magnetic anisotropy, and the value of the anisotropy (62.77 × 103 erg/g) is associated with the reluctant the dipoles are to experience the annihilation of their magnetically linked arrangement underneath the reverse external field, while the SFD measures the M–H hysteresis rectangularity. The outcomes show that the CoFe2O4’s magnetic characteristics are preserved in the investigated sample. The CoSmFe-G nanosample may thus be easily isolated from an aqueous solution using an external magnetic field because of its enhanced magnetic performance (Table 4).
The variation in the value of Ms that has been observed could be caused by the dopant’s nature as well as composition, in addition to the size, shape, and arrangement of the resulting particles. Similarly, the samples’ reported Ms values are explained by lattice defects, the relationship between magnetic super exchange between the A- to B-sites, with the random magnetic orientation that results from the spins on the doped Co-ferrite. Moreover, it is apparent the fact the Ms variation caused by the substitution of Sm3+ and the grain size modification are fairly comparable. There are reports that the decrease in magnetization is caused by the smaller grain size. The reduced grain size inhibits domain migration, which raises the HC in the substituted compounds. These findings demonstrate a significant reduction in the saturation magnetization of the nanocomposite relative to the CoFe2O4 nanoparticles. This can be attributed to the small amount of magnetic nanoparticles utilized in the nanocomposite’s synthesis as well as the presence of graphene, which lowers the magnetization moment. On the other hand, our sample’s saturation magnetization is moderate. The intense attraction of the magnetic nanoparticles which create spherical clusters upon the graphene surface explains the cause of the increase in coercivity in our sample. In the circumstance of the magnetic regaining of the Graphene-based Sm-doped CoFe2O4 following the removal of heavy metals from water, such magnetic behavior has become desirable for numerous uses demanding strong magnetic signals under modest applied magnetic fields.
3.4 Zeta potential
The purpose of zeta potential is to provide information about a suspension’s stability. The electrokinetic potential of liquid–liquid or solid–liquid colloidal dispersions is described by the Zeta Potential [77]. When charged colloidal dispersions are introduced into a zeta cell for a zeta potential study, the particles move in the direction of the electrode that has the opposite charge to the particle when an external electric field is applied [78]. The zeta potential is then determined using their velocity, or electrophoretic mobility, in the presence of an electric field. The amount of OH− and H+ ions on the surface determines the chemical characteristics of the suspended materials. Most of the atoms on the surface of nanoparticles are unsaturated, which means that they readily draw in additional atoms. Nanomaterials were identified by their rapid and potent adsorption rates [38].
A point of zero charge, or PZC, is the pH level at which the net surface charge of the adsorbent is equal to zero. This concept is the result of growing interest in the pH level of the solution during adsorption. pH has attracted a lot of attention because certain chemicals’ adsorption depends heavily on it. The PZC value is considered a characteristic of an adsorbent. At PZC, the charge covering the adsorbent’s surface is described by the ion present on the outermost layer of the particles (adsorbent) composition-like image [19, 38].
Figure 10 illustrates the zeta potential study for CoSmFe-G at 25 °C in an aqua solution at different pH (2, 7, 10, and 11) respectively. This study showed PZC at a pH about 7.2 that indicates CoSmFe-G will have positive charges at pH values less than 7.2, and negative charges at pH values greater than 7.2. This will undoubtedly aid in comprehending the mechanism of Pb2+ binding to CoSmFe-G. This predicts a different adsorption efficiency for Pb2+ ions in pH-varying solutions. Presumably, the cation exchange mechanism will be predominant at lower pH values (where the adsorbents are positively charged), and at higher pH values (where the adsorbents are negatively charged), electrostatic forces will primarily drive the removal of Pb2+.
3.5 Applications
3.5.1 Antibacterial activity
When evaluating the antibacterial effectiveness of graphene-based Sm-doped Cobalt ferrite CoSmFe-G, a wide variety of experimental factors should be considered, including the kind and physicochemical characteristics of the antibacterial agent, bacterial morphology and category, and the application setting (in vitro/in vivo). antibacterial mechanisms: the physicochemical interactions among the nanosample and harmful germs are often what determine how effective nanomaterials are in killing bacteria [68, 79]. The primary barrier and a defining characteristic of the sample’s antibacterial activity is the cell envelope. While Gram-negative bacteria (E. coli, P. aeruginsa, and S. typhimurium) have a weak peptidoglycan layer (5–10 nm) but include an outside cellular membrane made of (7.5–10 nm) lipopolysaccharides, Gram-positive bacteria (S. aureus, B. cereus, and S. faecalis) possess a thick cell wall made of a (30–100 nm) peptidoglycan layer. By keeping hazardous chemicals out, the outer cellular membrane gives bacterial cells the necessary defense and stability against hostile surroundings. DNA, lipids, as well as proteins inside cells are damaged by the highly oxidizing ROS produced by the photocatalytic process [80, 81]. ROS besides, nano-antibacteria have been demonstrated to be the important factors determining the antibacterial action. The inhibition zones of the CoSmFe-G nanosample against various strains are shown in Fig. 11. Compared to the literature shown in Table 5, this investigated sample has strong antibacterial properties. The antibacterial sample has some selectivity in the suppression of bacteria, which results from variations in the bacterial cell architecture. The prepared sample is deemed suitable as an antibacterial agent by the standard and is a potentially useful and effective antibacterial substance. Roughness surface and defects of graphene-based nanoparticles can sever the bacterial cell membrane, causing pores to develop, changes in the bacterial osmotic pressure, and cell death.
3.5.2 Adsorption study
3.5.2.1 Effect of experimental parameters (pH, and contact time)
Figure 12a shows the relationship between heavy metal ion adsorption and pH solution. According to the graph, the removal efficiency rises with pH in the range of 2 to 8, with (pH 7) being the exception [87]. The hydrolysis products of Fe2+ are FeOH+, Fe(OH)2, and Fe(OH)3, and the primary interactions in the adsorption process are electrostatic. When the pH changes, these products alter. Therefore, the electrostatic interactions between metal ions and the surface charge are what cause heavy metal ion adsorption. Lower pH values are associated with less heavy metal absorption, and this may be attributed to competition among the heavy metal ions (Pb2+) and H+ occupying adsorbent active sites. Furthermore, H3O+ is more mobile than a metal ion [88]. The protonation that occurs in the hydroxyl group diminishes because the pH of the aqueous solution rises. Because of this, there are additionally active adsorbent sites accessible to remove heavy metals from solutions [66]. It is possible to ascribe the metal ion adsorption observed in the CoSmFe-G nanosample to exterior adsorption, and since the lead ion may easily access active adsorption sites, a fast uptake will occur. At (pH 7), the greatest adsorption efficiencies were 99.8%. By varying the contact duration between CoSmFe-G nanoparticles as well as the metal ion solutions over 10 to 60 min while employing 0.1 g of adsorbent with an initial metal value of 2 ppm and (pH 7), the kinetics of (Pb2+) adsorption are investigated. Figure 12b presents the observed removal rates removal efficiency of (Pb2+). Due to the more active sites on the adsorbent’s surface, the removal effectiveness increases with initial contact time. All these locations are occupied at the ideal moment, and the removal efficiency is constant after 50 min (99.8%). According to the prior information, the ideal pH of the solution and the recommended contact time for heavy metal ion adsorption are 7 and 50 min, respectively.
Only 0.1 g of graphene combined with inexpensive magnetic elements was used to remove 99.8% of the (Pb2+) from wastewater. The primary innovation of this study is this as indicated in Table 6, many authors have created graphene nanocomposite materials for the elimination of heavy metals. The table shows that the studied samples had better removal efficiencies than most of the other adsorbents, which qualifies the CoSmFe-G nanosample as an effective adsorbent for the removal of Pb metal ions. CoSmFe-G nanosample’s ability to uptake heavy metal ions is compared in Table 6 to that of other adsorbents from the literature.
3.5.2.2 Adsorption mechanism
Large-surface area graphene that has excellent conductivity, ionic mobility, as well as exceptional mechanical flexibility could serve as a useful auxiliary material for enhancing the adsorption capabilities of adsorbents. We suggested a potential (Pb2+) adsorption by CoSmFe-G nanosample based on the prior discussion, as shown in Fig. 12. (Pb2+) ions are chemically and/or electrostatically attracted to the CoFe2O4 surface when an adsorbent is added to a solution that contains (Pb2+) ions. The use of graphene improves the surface area provided by the adsorbent, increasing the lead ion absorption sites. Two isotherm models were looked into to better understand how solutes sorb into an adsorbent forming an aqueous solution. Both Langmuir and Freundlich isotherm models are examined. (R2) is the regression coefficient, which indicates the factor supporting the isotherm model. If the material that absorbs is predicted as a monolayer, a finite number of comparable sites over the surface related to the adsorbent follow Langmuir adsorption isotherm. The provided equation is followed by the Langmuir adsorption model [89].
where Ce is the optimal concentration of the metallic ion (mg/L), qe as well as qm (mg/g) are the adsorption capacities at equilibrium as well as maximum adsorption, while Kl (L/mg) represents the affinity binding constant. The Freundlich isotherm approach with a heterogeneous surface over the adsorbent is followed if the adsorption comprises a multilayer. The Freundlich isotherm model is supported by the following equation [39].
where qe stands for equilibrium adsorption capacity, qm (mg/g) for maximum adsorption, and Kl (L/mg) for affinity binding constant. The adsorption capacity and intensity are represented, respectively, by the physical constants Kf and n.
The obtained data indicates that the experiment’s outcomes suit the two isotherm models rather well. For the Langmuir and Freundlich isothermal, the regression coefficient (R2) estimations are 0.999 and 0.866, respectively, as shown in Fig. 13a and b. Because the Langmuir isotherm model and the (Pb2+) adsorption were a good fit, the adsorption may be classified as monolayer type which results from homogeneous surface group distribution of CoSmFe-G nanosample.
3.5.2.3 Adsorption’s kinetic investigation
The following equations were used to analyze the kinetic performance over the adsorption process using three kinetic models: the inter-particle one, the pseudo-first, and the pseudo-second [18, 79, 90, 91].
Model of pseudo-first-order: The formula is:
The pseudo-second-order model is given by:
Model for the diffusion of inter particles between them:
where qe, as well as qt, are the adsorption capacities in equilibrium and at the specified time t, and k1, k2, and k3 are pseudo-first-order, pseudo-second-order, and inter-particle diffusion constants, respectively.
As demonstrated in Fig. 14a–c the observed adsorption data was properly linearly fitted with the pseudo-first, pseudo-second, as well as inter-particle models. After 40 min of contact time, a condition of equilibrium throughout the adsorption capacity had been reached, according to the fits. Additionally, there were two methods used to remove (Pb2+) ions: (a) (Pb2+) diffusion into the examined nanoparticles [90] and (b) (Pb2+) ion sorption into the CoSmFe-G nanosample. The adsorption phenomena are best described by the pseudo-second-order kinetic idea, which outperforms the other two models [92]. The inter-particle diffusion model had a very poor fit, proving that this form of secondary diffusion is not what regulates the adsorption of heavy metals.
4 Conclusion
Here, a straightforward, low cost and easy method for producing a high-performance material for antibacterial and heavy metal removal applications a (CoSm0.025Fe1.975O4)0.9G0.1 nano sample has been implemented. Graphene presence is confirmed after chemical and thermal treatment in a single-layered graphene structure by Raman spectroscopy and the integration of Sm-doped CoFe2O4 nanoparticles into the graphene resulted in the formation of 90 nm cluster-like formations. The sample exhibits typical soft ferrimagnetism behavior; with saturation magnetization in the 15 kOe region when exposed to an external field. Zeta potential allocates that PZC was found at a pH about 7.2. The inhibition zones were found to vary between 12 and 15 mm because the roughness and defects of graphene-based nanoparticles can rupture the bacterial cell membrane, causing holes to develop, changes in the bacterial osmotic pressure, and cell death.
The information on adsorption states that for heavy metal ion adsorption (99.8%), the optimal pH of the solution is 7, and the suggested contact period is 50 min. It is important to note that while functionalizing the surface of graphene-based NMs may significantly improve their biocompatibility, it is crucial to assess the long-term resilience of the surface coating. By carefully considering the unique properties of graphene, Sm, and G-based Sm-doped Cobalt ferrites, this study may guide developing practical and efficient water disinfection solutions.
Data availability
Data shall be made available upon request.
References
M. Stoia, M. Stefanescu, T. Dippong, O. Stefanescu, P. Barvinschi, J. Sol–Gel Sci. Technol. 54, 49–56 (2010)
M. Tefnescu, T. Dippong, M. Stoia, O. Tefnescu, J. Thermal Anal. Calorim. 94, 389–393 (2008)
T. Dippong, E.A. Levei, C. Leostean, O. Cadar, J. Alloys Compd. 868, 159203 (2021)
M.N. Akhtar, M. AzharKhan, M. Ahmad, M.S. Nazir, M. Imran, A. Ali, A. Sattar, G. Murtaza, J. Magn. Magn. Mater. 421, 260–268 (2017)
T. Dippong, E.A. Levei, F. Goga, O. Cadar, Mater Charact 172, 110835 (2021)
T. Dipponga, E.A. Leveib, D. Tolomanc, L. Barbu-Tudoranc, O. Cadar, J. Anal. Appl. Pyroly. 158, 105281 (2021)
T. Dippong, D. Toloman, M. Dan, E.A. Levei, B. Mare, J. Alloys Compd. 913, 165129 (2022)
T. Dippong, E.A. Levei, I.G. Deac, O. Cadar, Nanomaterials 11, 3455 (2021)
M.N. Akhtar, M. Saleem, M. AzharKhan, J. Phys. Chem. Solids 123, 260–265 (2018)
C.M. Park, Y.M. Kim, K.H. Kim, D. Wang, C. Su, Y. Yoon, Chemosphere, vol. 221 (Elsevier Ltd, Amsterdam, 2019), pp.392–402
K.A. Elsayed, H. Imam, M.A. Ahmed, R. Ramadan, Opt. Laser Technol. 45, 495–502 (2013)
C.-H. Yu, G.-Y. Chen, M.-Y. Xia, Y. Xie, Y.-Q. Chi, Z.-Y. He, C.-L. Zhang, T. Zhang, Q.-M. Chen, Q. Peng, Colloids Surf. B 191, 111009 (2020)
I. Anastopoulos, A. Bhatnagar, E.C. Lima, J. Mol. Liquids 221, 954–962 (2016)
S. Hemasankari, S. Priyadharshini, D. Thangaraju, V. Sathiyanarayanamoorthi, N. Alsdran, M. Shkir, Physica B 660, 414870 (2023)
M. Hashim, M. Raghasudha, S.S. Meena, J. Shah, S.E. Shirsath, S. Kumar, D. Ravinder, P. Bhatt, A. Muddin, R. Kumar, R.K. Kotnala, J. Magn. Magn. Mater. 449, 319–327 (2018)
F. Falsafi, B. Hashemia, A. Mirzaeia, E. Faziob, F. Nerib, N. Donatoc, S.G. Leonardic, Ceram. Int. 43(1), 1029–1037 (2017)
A. S. Shafaay and R. Ramadan, J. Supercond. Nov. Magn. (2023)
M.M. Arman, R. Ramadan, J. Mater. Sci. 34, 17 (2023)
R. Ramadan, A.S. Shafaay, J. Water Process. Eng. 54, 103958 (2023)
M.N. Akhtar, S. Makhdoom, M.A. Baqir, M. Yousa, M. Azhar Khane, K.M. Batoo, S. Hussain, Ceram. Int. 48, 29561–29571 (2022)
R. Ramadan, F.N. Almutairi, G.A. Alzaidy, Ceram. Int. (2023)
M.N. Akhtar, A.B. Sulong, M. Ahmad, M. AzharKhan, A. Ali, M.U. Islam, J. Alloys Compd. 660, 486–495 (2016)
W.S. Hummer, R.E. Offeman, J. Am. Chem. Soc. 90, 1339 (1958)
M. Siyar, N. Khan, A. Maqsood, M. Younas, M. Daud, Int. J. Sci. Eng. Res. 5(7), 376 (2014)
Z. Lin, G.H. Waller, Y. Liu, M. Liu, C. Wong, Nano Energy 2, 241–248 (2013)
A.K.R. Rai, T.V. Thi, J. Gim, J. Kim, Mater Charact 95, 259–265 (2014)
E.E. Ateia, R. Ramadan, A.S. Shafaay, Appl. Phys. A 126, 222 (2020)
E.E. Ateia, M.K. Abdelamksoud, M.M. Arman, Appl. Phys. A 125, 516 (2019)
M. Rostami, R. Mostafa Zamani, K.M. Aghajanzadeh, H. Danafar, J. Pharm. Investig. 48, 657–664 (2018)
R. Ramadan, N. Shehata, Desalin. Water Treat. 227, 370–383 (2021)
M. Yousaf, M. Akbar, M.A.K. Yousaf Shah, A. Noor, Y. Lu, M.N. Akhtar, N. Mushtaq, E. Hu, S. Yan, B. Zhu, Ceram. Int. 48(19), 28142–28153 (2022)
Y. Lu, M. Yousaf, M.N. Akhtar, A. Noor, M. Akbar, M.A.K. Yousaf Shah, S. Yan, F. Wang, Ceram. Int. 48(2), 2782–2792 (2022)
K.K. Patankar, D.M. Ghone, V.L. Mathe, S.D. Kaushik, J. Magn. Magn. Mater. 454, 71–77 (2018)
A.M. Ismail, R. Ramadan, M.M. El-Masry, J. Aust. Ceram. Soc. 59(2), 333–341 (2023)
H. Imam, K.D. Elsayed, M.A. Ahmed, R. Ramdan, Opt. Photon. J. 2, 73–84 (2012)
S.R. Naik, A.V. Salker, J. Mater. Chem. 22, 2740–2750 (2012)
D.M. Ghone, V.L. Mathe, K.K. Patankar, S.D. Kaushik, J. Alloys Compd. 739, 52 (2018)
M.A. Ahmed, S.T. Bishay, S.M. Abd-Elwahab, R. Ramadan, J. Mol. Liquids 240, 604–612 (2017)
M.A. Ahmed, S.T. Bishay, R. Ramadan, J. Nanosci. Nanotechnol. 9(2), 064–074 (2015)
R. Ramadan, V. Uskoković, M.M. El-Masry, J. Alloys Compd. (2023). https://doi.org/10.1016/j.jallcom.2023.170040
M.K. Ahmed, R. Ramadan, S.I. El-dek, V. Uskokovi, J. Alloys Compd. 801, 70–81 (2019)
R. Ramadan, A.M. Ismail, J. Inorg. Organomet. Polym. Mater. (2023)
R. Ramadan, G.A. Alzaidy, F.N. Almutairi, V. Uskoković, Appl. Phys. A 129(11), 1–24 (2023)
G. Xi, T. Zhao, L. Wang, C. Dun, Y. Zhang, Physica B 534, 76–82 (2018)
M. George, T.L. Ajeesha, A. Manikandan, A. Anantharaman, R.S. Jansi, E.R. Kumar, Y. Slimani, M.A. Almessiere, A. Baykal, J. Phys. Chem. Solids 153, 110010 (2021)
J. Bennet, R. Tholkappiyan, K. Vishista, N.V. Jaya, F. Hamed, Appl. Surf. Sci. 383, 113–125 (2016)
E.E. Ateia, A.A. El-Bassuony, G. Abdlatif, F.S. Soliman, J. Mater. Sci. 28, 241–249 (2017)
E. Hema, A. Manikandan,·P. Karthika,·S. Arul Antony,·B.R. Venkatraman, J. Supercond. Nov. Magn. (2015)
N. Sanpo, J. Wang, C.C. Berndt, J. Nano Res. 22(2013), 95–106 (2013)
B. Jeevanantham, Y. Song, H. Choe, M.K. Shoban, Mater. Lett. 12, 100105 (2021)
K. Nadeemn, M. Shahid, M. Mumtaz, Progr. Natl. Sci. 24, 199–220 (2014)
C. Xia, T. Ren, R. Darabi, M. Shabani-Nooshabadi, J. Kleme, C. Karaman, F. Karimi , Y. Wu, H. Kamyab, Y. Vasseghian, S. Chelliapan, Energy (2023)
M.M. Rashad, R.M. Mohamed, H. El-Shall, J. Mater. Process. Technol. 198(1–3), 139–146 (2008)
R. Ramadan, Appl. Phys. A (2023)
R.C. Che, L.M. Peng, X.F. Duan, Q. Chen, X.L. Liang, Adv. Mater. 16, 401–405 (2004)
L. Zhou, H. Deng, J. Wan, J. Shi, T. Su, Appl. Surf. Sci. 283, 1024 (2013)
P. Sivakumar, R. Ramesh, A. Ramanand, S. Ponnusamy, C. Muthamizhchelvan, J. Alloys Compd. 563, 6–11 (2013)
H. Kavas, N. Kasapoğlu, A. Baykal, Y. Köseoğlu, Chem. Pap. 63, 450–455 (2009)
Y. Fu, H. Chen, X. Sun, X. Wang, AIChE J. 58, 3298–3305 (2012)
S. Briceño, C. Reinoso, Environ. Res. (2022)
H. Xia, D. Zhu, Y. Fu, X. Wang, Electrochim. Acta 83, 166–174 (2012)
M. Wall, PhD, Thermo Fisher Scientific, Madison, WI, USA, http://thermofisher.com/energy
I. Shakir, P.O. Agboola, S. Haider, Ceram. Int. 47(20), 28367–28376 (2021)
Y. Fu, H. Chen, X. Sun, X. Wang, Appl. Catal. B 111–112, 280–287 (2012)
R. Ramadan, Appl. Phys. A (2019)
R. Ramadan, M.K. Ahmed, J. Alloy. Compd. 856, 157013 (2021)
M.M. El-Masry, M. El-Shahat, R. Ramadan, R.M. Abdelhameed, J. Mater. Sci. 32(13), 18408–18424 (2021)
M. Kaur, M. Dhiman, A. Sudhaik, P. Raizada, P. Singh, S. Gautam, Mater. Today Proc. (2023)
K.L. Routray, S. Saha, D. Behera, Mater. Sci. Eng. B (2020)
M. Shaterian, A. Aghaei, M. Koohi, M. Teymouri, A. Mohammadi-Ganjgah, Polyhedron. (2020)
L. Zou, X. Xiao, C. Chu, B. Chen, Sci. Total Environ. (2021)
S.A. Al Kiey, R. Ramadan, M.M. El-Masry, Appl. Phys. A 128, 6 (2022)
F. Sharifianjazi, M. Moradi, N. Parvin, A. Nemati, A.J. Rad, N. Sheysi, A. Abouchenari, A. Mohammadi, S. Karbasi, Z. Ahmadi, A. Esmaeilkhanian, M. Irani, A. Pakseresht, S. Sahmani, M. ShahediAsl, Ceram. Int. 46(11), 18391–18412 (2020)
S. Velho-Pereira, A. Noronha, A. Mathias, R. Zakane, V. Naik, P. Naik, A.V. Salker, S.R. Naik, Mater. Sci. Eng. C 52, 282–287 (2015)
M. Hashim, N. Boda, A. Ahmed, S.K. Sharma, D. Ravinder, E. Sumalatha, A. Ul-Hamid, M.M. Ismail, M. Chaman, S.E. Shirsath, R. Kumar, S. Kumar, S.S. Meena, M. Nasir, Appl. Phys. A 127, 526 (2021)
S. Brice, C. Reinoso, Environ. Res. 212, 113470 (2022)
https://particletechlabs.com/analytical-testing/zeta-potential-analysis.
X. Wang, B. Cheng, L. Zhang, J. Yu, Y. Li, J. Colloid Interface Sci. 612, 598–607 (2022)
G. Ma, Y. Chen, L. Li, D. Jiang, R. Qiao, Y. Zhu, Mater. Lett. 131, 38–41 (2014)
B. Omran, K.H. Baek, Environmental Pollution, vol. 298 (Elsevier, Amsterdam, 2022)
E. Avcu, F.E. Bastan, M. Guney, Y. Yildiran Avcu, M.A. Ur Rehman, A.R. Boccaccini, Acta Biomater. 151, 1–44 (2022)
K. Balakrishnan, S. Sreekumar, K. Arumugam, P.K. Gupta, Biocatal. Agric. Biotechnol. 57, 103114 (2024)
S. Gnanama, J. Gajendiranb, R. Ashokkumar, K. Ramachandrand, J. Ramana Ramya, J. Mol. Struct. 1229, 129846 (2021)
E.M. Ahmed, H. Isawi, M. Morsy, M.H. Hemida, H. Moustafa, Surf. Interfaces 39, 102980 (2023)
S. Raissi, A. Najjari, M.K. Younes, J. Matchemphys. 308, 128206 (2023)
S.O. Aisida, K. Ugwu, A. Agbogu, I. Ahmad, M. Maaza, F.I. Ezema, Hybrid Adv. 3, 100049 (2023)
M.A. Almessiere, S. Güner, Y. Slimani, B.R. Jermy, M. Sertkol, N. Taskhandi, A.D. Korkmaz, A. Baykal, Ceram. Int. 47(17), 25131–25141 (2021)
V. Banihashemi, M.E. Ghazi, M. Izadifard, Physica B (2021)
R. Ramadan, M.M. El-Masry, J. Austral. Ceram. Soc. (2023)
Y.H. Hou, Y.L. Huang, S.J. Hou, S.C. Ma, Z.W. Liu, Y.F. Ouyang, J. Magn. Magn. Mater. 421, 300–305 (2017)
A. Hashhash, I. Bobrikov, M. Yehia, M. Kaiser, E. Uyanga, J. Magn. Magn. Mater. (2020)
R. Ramadan, A.M. Ismail, J. Inorg. Organomet. Polym. Mater. 32(3), 984–998 (2022)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Rania Ramadan: Conceptualization, methodology, formal analysis, data curation, investigation, visualization, writing, review. Amira S. Shafaay: Conceptualization, methodology, formal analysis, data curation, investigation, visualization, writing, review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramadan, R., Shafaay, A.S. Graphene-based Sm-doped Co-ferrite for environmental applications. J Mater Sci: Mater Electron 35, 1331 (2024). https://doi.org/10.1007/s10854-024-12980-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12980-z