Abstract
Our study sheds light on the role of secondary phases in optimizing the magnetic properties of LSMO, offering valuable insights into the relationship between impurity phases, Curie temperature (TC), and magnetic entropy change. To achieve this goal, the Mn, Ni, and Mo elements were individually incorporated into the LSMO matrix to the formation of secondary phases. The solid-state reaction method was employed to create composite materials of (0.8)La0.67Sr0.33MnO3 + (0.2)A (A=Mn, Ni, and Mo). XRD and SEM results confirmed the successful formation of impurity phases within the main LSMO phase. From temperature-dependent magnetization (M(T)) measurements, it was determined that the TC of the samples approach towards room temperature with the existence of impurity phases. Additionally, the relationship between electron bandwidth (W) and TC was examined. The lowest TC value was observed for the lowest value of W. Besides these, the effect of the formation of impurity phases on the maximum magnetic entropy change (\(-\Delta {S}_{M}^{max}\)) value is examined, and it is seen that impurity phases cause a decrease in the \((-\Delta {S}_{M}^{max})\) values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent focus on magnetic cooling (MC) systems as eco-friendly alternatives to conventional cooling methods has surged due to their potential to reduce environmental impact while providing efficient cooling solutions. The discovery of the magnetocaloric effect (MCE) in Gd and Gd-based alloys sparked initial research interest in this field, laying the groundwork for subsequent advancements [1,2,3]. Further progress in MC system development has been propelled by breakthroughs like the exploration of manganite perovskites, known for their cost-effectiveness and ease of production [4,5,6,7]. Manganite perovskites, such as La1-xAxMnO3, have garnered significant attention due to their intriguing properties, including a wide variation in the paramagnetic-ferromagnetic phase transition temperature, TC, depending on the valency and concentration of elements in the A–site [8, 9]. Notably, previous studies have demonstrated the feasibility of optimizing TC to room temperature by manipulating the concentrations of elements with varying valences in La1-xAxMnO3manganites [10,11,12,13]. However, achieving TC at room temperature alone is insufficient for practical applications. Materials intended for MC systems require a TC near room temperature, high MCE over a wide temperature range, and high relative cooling power (RCP) at low magnetic fields. Consequently, recent research efforts have focused on simultaneously achieving these desired properties. Initial investigations primarily involved doping La in the A-site with different concentrations of similar elements, yielding promising results [14,15,16]. Subsequent studies extended this approach to simultaneously doping elements with similar or different valences, in varying concentrations, further enhancing the magnetic properties [17,18,19]. More recently, attention has shifted towards composite materials to broaden the MCE effect's temperature range. Composite materials, formed by combining samples with differing magnetic properties in various ratios, have shown promise [20]. It is widely acknowledged that the magnetic interactions occurring on the surfaces of the grains in the samples and within the grain boundaries are more intricate than those occurring within the grains themselves [21]. Therefore, it has been observed that in some composite materials formed with a manganite and an insulating/conductive phase, the mixture of two different phases changes the magnetic properties of the parent sample [22, 23]. In particular, it has been revealed in the studies that the secondary phases located at the grain boundaries of the manganite work as an energy barrier [24]. Therefore, additive secondary phases into the manganites should affect the MCE and TC of the parent manganite.
It is well established in the literature that the La0.67Sr0.33MnO3 manganite exhibits a high TC well above room temperature [25], yet its MCE is also known as insufficient for practical technological applications. Previous studies investigating composite materials have explored various approaches to enhance the MCE and TC of manganite-based systems. K. Das et al. prepared a manganite–manganite composite material by adding Pr0.67Ca0.33MnO3 to the La0.67Sr0.33MnO3 compound, which has a TC well above room temperature. They successfully optimized the TC value of the composite material to around room temperature and reported an increase in the RCP [26]. Ali et al. reported that they prepared a composite material by adding a secondary phase of Co3O4 to the La0.7Sr0.3MnO3 compound, which has a TC well above room temperature, and successfully lowered the TC value to around room temperature. However, they did not observe a significant alteration in the magnetic entropy change (ΔSM) values [27]. T.P. Gavrilova et al. investigated the magnetic and magnetocaloric properties of (1 − x)La0.7Sr0.3MnO3/xNaF composites and they found that increasing the amount of NaF in the composite led to a decrease in the TC value along with an increase in the RCP [28].
Building upon our previous study, in which we observed that the La0.67Sr0.33MnO3 manganite exhibited a TC value significantly above room temperature, the current research seeks to enhance and optimize its magnetic properties [29]. However, we state that this current research differs from the findings presented in reference [29], except for those related to the parent compound, as it seeks to undertake a distinct investigation aimed at optimizing the magnetic properties of the aforementioned parent compound. To achieve this objective, we prepared manganite composite materials (0.8)La0.67Sr0.33MnO3 + (0.2)A (A=Mn, Ni, and Mo) and conducted a comprehensive investigation of their structural, magnetic, and magnetocaloric properties. The selection of Mn, Ni, and Mo elements aims to optimize the magnetic, structural, and electronic properties of the resulting composite materials. Mn, Mo, and Ni are transition metals capable of exhibiting different oxidation states, which critically influence the compound's magnetic and electrical characteristics. Mn can display various magnetic properties depending on its crystal structure, such as ferromagnetic, antiferromagnetic, or ferrimagnetic behavior [30]. Nickel naturally enhances the magnetic behavior of the composite material as a ferromagnetic element [31]. Meanwhile, Mo typically exhibits paramagnetic behavior, thereby diversifying the electronic structure of the composite material and contributing to different magnetic behaviors [32]. The combination of these three elements with the LSMO compound enables the diversification of the composite material's magnetic, electronic, and structural properties, ultimately optimizing its magnetic properties around room temperature. This approach presents a significant strategy for ensuring the desired performance of LSMO compound in targeted applications.
2 Experimental process
La0.67Sr0.33MnO3 samples were synthesized by the sol–gel method. In this method, an appropriate stoichiometric amount of lanthanum (III) nitrate hexahydrate (La(NO3)3·6H2O; 99.99% purity, Alfa Aesar), strontium nitrate (Sr(NO3)2; 99.97% purity, Alfa Aesar), and manganese(II) nitrate tetrahydrate (Mn(NO3)2·4H2O; 98.5% purity, Merck) was used. A detailed explanation of the sol–gel method can be found in our previous study [33]. The solid precursor obtained was finely ground to achieve a homogeneous powder. For the synthesis of composite materials, La0.67Sr0.33MnO3 (0.8 g) manganite and each of the Mn (Alfa Aesar; 99.95% purity), Ni (Alfa Aesar; 99.9% purity), and Mo (Aldrich; 99.9 + % purity) elements (0.2 g) that were selected by our research objectives were meticulously mixed. To ensure the uniform dispersion of these elements within the composite, the mixture underwent rigorous grinding, a crucial step in achieving homogeneity. Following the grinding process, we obtained composite materials containing the desired proportions of the La0.67Sr0.33MnO3 manganite and the added elements. Subsequently, 1 g of the powder composite materials was pressed into tablets with a thickness of 4 mm and a diameter of 1.3 cm under a pressure of 6 tons using a hydraulic press. These tablets were sintered in an adjustable high-temperature cylindrical furnace at 1000 °C for 24 h under an air atmosphere.
To simplify nomenclature in this paper, we have abbreviated the composite names as follows: La0.67Sr0.33MnO3 is denoted as LSMO, (0.8)La0.67Sr0.33MnO3 + (0.2)Mn is represented as LSMO–Mn, (0.8)La0.67Sr0.33MnO3 + (0.2)Ni is abbreviated as LSMO–Ni, and (0.8)La0.67Sr0.33MnO3 + (0.2)Mo is indicated as LSMO–Mo.
X-ray diffraction pattern (XRD) studies were carried out to find the crystal symmetries, lattice parameters, phase purity, and unit cell volume of the samples. The surface morphology studies were carried out by using a field emission scanning electron microscope (FESEM, JEOL, JSM 5800) to reveal the properties of the granular structure (grain size, grain shape, grain boundaries, and porosities) on the surface of the samples. To check the suitability of the produced samples to the targeted samples, energy-dispersive X–ray spectroscopy (EDS) studies were carried out simultaneously during the SEM analyses. For investigating magnetic behavior and determining TC of the composites, the temperature dependence of magnetization (M(T)) both under zero–field–cooling (ZFC) and field–cooling (FC) modes were measured from 5 to 370 K using the Quantum Design Physical Properties Measurement System (PPMS) with a closed–cycle helium cryostat. During the ZFC measurement process, the sample was cooled from room temperature to 10 K without being exposed to any magnetic field, and the magnetization was recorded. Then, a 100 mT magnetic field was applied to the sample and heated to room temperature. In the FC measurement process, the sample was cooled to 10 K without removing the 100 mT magnetic field applied to the sample at room temperature, and the magnetization was recorded again. After the TC of the composites was determined, magnetization (M(H)) measurements were performed against the magnetic field at 4 K intervals in the temperature regions above and below TC for each composite. Magnetic entropy changes (–ΔSM) were calculated indirectly from M(H) data by using the following equation;
For easier calculation, the above integral is approximated with a summation and the derivative in the integral with the finite difference form with discrete magnetization at separate temperatures and applied fields, as follows;
where \({\left(\Delta {S}_{M}\right)}_{i}\) is the magnetic entropy change at a temperature Ti. Mi and Mi+1 are the experimental magnetizations obtained at the temperatures Ti and Ti+1 under the magnetic field \(\Delta\) Hj.
3 Results and discussions
3.1 X-ray diffraction (XRD) analysis
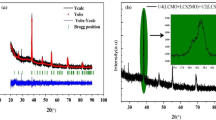
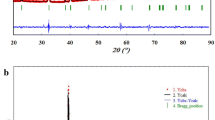
This study aims to decrease the TC of the LSMO sample, which currently exhibits a TC significantly higher than room temperature, by introducing various doped elements as secondary phases. In our previous study, the LSMO manganite with TC exceeding room temperature was synthesized [29]. To optimize the TC of the LSMO sample, we introduced certain metal additives to create secondary phases in the main phase system. If secondary phases related to additive elements are identified in the XRD analysis, distinct from the LSMO sample, we can obtain composite material. The XRD patterns of the composite materials produced and their analysis are some of the most important parts of the evidence showing whether the target in the study has been achieved. Therefore, to prove the existence of any pattern formation involving secondary phases as well as the characteristic XRD patterns of the LSMO manganite, XRD analysis was performed. The XRD patterns and detailed Rietveld refinements of the samples are presented in Fig. 1a–d [34]. As was given in our previous study, the LSMO sample crystallized in the perovskite structure with a trigonal (\(R\overline{3 }c\)) space group [29]. LSMO–Mn, LSMO–Ni, and LSMO–Mo composites also were crystallized in the same crystal structure. It has been observed that the XRD patterns of LSMO–Mn, LSMO–Ni, and LSMO–Mo composite are different than those of LSMO. In the XRD pattern of LSMO–Mn and LSMO–Ni, extra Mn3O4 and NiO peaks were observed, respectively. This indicates the absence of any reaction between metal elements and LSMO. The observed intensities of Mn3O4 and NiO peaks are prominent in concordance with the 20% content of Mn and Ni in the LSMO matrix. According to this, it can be concluded that the LSMO–Mn and LSMO–Ni consisted of two separate phases (LSMO + Mn3O4 and LSMO + NiO). As we mentioned before, the LSMO compound was synthesized via the sol–gel method and annealed at 600 °C, resulting in the formation of a crystal structure close to the perovskite. The LSMO–Mn and LSMO–Ni composite materials were obtained by separately adding Mn and Ni elements to the LSMO compound through the sol–gel method, followed by the solid-state reaction method, resulting in powders. During this process, no chemical reaction occurred between LSMO and the additives; however, they were physically mixed. The mixture was then pressed into tablets and sintered at a high temperature (1000 °C) for 24 h, promoting densification and initiating the formation of crystal structures. However, the main reason for the formation of impurity phases observed in LSMO–Mn and LSMO–Ni composite materials may be the incomplete integration of the added Mn and Ni elements into the crystal structure of LSMO. This limitation could arise from the limited ability of Mn and Ni elements to directly integrate into the stable structure of the LSMO matrix. Additionally, the incomplete occurrence of chemical reactions between Mn, Ni elements, and LSMO due to the high sintering temperature and pressure may also play a role in the formation of these impurity phases.
Compared to the LSMO compound, the unit cell volume of the LSMO–Ni composite material decreased, while that of the LSMO–Mo composite material increased, owing to changes in the crystal lattice parameters. The presence of secondary phases in the LSMO–Ni and LSMO–Mo composite materials, resulting from Ni and Mo elements with different ionic radii, influenced the unit cell volume of the LSMO compound's perovskite structure. These secondary phases induced deformations in the crystal structure, thereby altering the unit cell volume. Such deformations may stem from the large or differing ionic radii of the additives (Ni, Mo), or their inability to fully integrate into the crystal structure. Moreover, the secondary phases formed by the added additives within the LSMO composite may disrupt the crystal structural harmony of the LSMO perovskite structure within the composite. Consequently, variations in the crystal structural properties of the perovskite structure, such as symmetry or cell parameters, could occur. These alterations are likely associated with certain distortions or deformations in the crystal structure due to the presence of impurity phases. Additionally, the existence of impurity phases may impact mechanisms such as the displacement of atoms within the composite or the creation of defective regions in the perovskite structure. Hence, the smaller ionic radius of Ni could result in a decrease in the unit cell volume in the LSMO–Ni composite, while the larger ionic radius of Mo might contribute to an increase in the unit cell volume in the LSMO–Mo composite.
The XRD pattern of LSMO–Mo composite is different from LSMO and the other two composites (LSMO–Mn and LSMO–Ni). Adding Mo at a rate of 20% in the LSMO system caused the formation of a new phase indexed as (La,Sr)MoO4 which is quite prominent in XDR patterns. Additionally, we have detected the presence of an extra impurity phase compatible with the diffraction pattern of the Mn3O4 phase. The formation of (La,Sr)MoO4 together with LSMO could be due to the reaction between the LSMO and Mo during the mixing or sintering process. When two phases interact chemically, they can generate two or more particular phases. It is also possible that Mo reacted with the oxygen present in the LSMO, leading to the formation of (La,Sr)MoO4. Also, the interaction between Mo and LSMO may cause a separation of certain La and Sr ions from the LSMO matrix, leaving behind vacant sites for Mn ions. These vacant Mn ions may facilitate the emergence of the Mn3O4 impurity phase. The reaction could have been favored by the high temperature and pressure conditions during the mixing process. Fragmentation of LSMO with the addition of Mo may be due to the chemical affinity between Mo and oxygen being stronger than that between LSMO and Mo, leading to the preferential formation of (La,Sr)MoO4. Furthermore, it is noticeable that (La,Sr)MoO4, which is present in a lower volume within the LSMO–Mo composite, exhibits more prominent XRD peak intensities compared to the LSMO manganite. The intensity of the resulting XRD peaks provides crucial information about the density of the crystal planes responsible for the diffraction, thereby revealing details about the quantity and quality of the present crystalline material [35]. When considering the (La,Sr)MoO4 phase and LSMO manganite, their XRD peak intensities diverge due to the distinctive characteristics of their crystal structures. (La,Sr)MoO4 exhibits a well-organized tetragonal structure with a precise arrangement of Mo and O atoms. This high level of organization facilitates efficient diffraction of X-rays, resulting in higher peak intensities. Conversely, LSMO possesses a perovskite structure that is more intricate and less orderly, causing reduced diffraction efficiency and, consequently, lower XRD peak intensities [35]. Moreover, the dissimilarity in atomic numbers between the elements present in the two phases contributes to the observed variation in XRD peak intensities. Mo found in (La,Sr)MoO4, possesses a higher atomic number than Mn in LSMO. As X-rays are more strongly scattered by atoms with higher atomic numbers, this disparity potentially accounts for the higher XRD peak intensities observed in (La,Sr)MoO4 [35]. Furthermore, another factor impacting the discrepancy in XRD peak intensities between (La,Sr)MoO4 and LSMO is the difference in their crystallite sizes (CS) (see Table 1). (La,Sr)MoO4 exhibits larger domains of crystalline structure, resulting in sharper and more intense XRD peaks. Conversely, LSMO possesses smaller crystalline domains, leading to broader and less intense XRD peaks [36].
Crystal structure, lattice parameters, unit cell volumes, Mn–O–Mn bond angles, Mn–O bond lengths, (CS), and goodness of fit (GOF) were obtained through the Rietveld refinement and they were presented in Table 1. While there was no significant change in the lattice parameters and unit cell volume of the parent LSMO and the LSMO–Mn samples, some changes were observed for LSMO–Ni and LSMO–Mo samples. Moreover, it was found that there were changes in the Mn–O–Mn bond angle for the LSMO–Mn and LSMO–Ni samples, while there was no significant change in the Mn–O bond lengths in the octahedral of the parent phase in all samples except LSMO–Mo. The data presented in Fig. 2 provide clear evidence that the modification in Mn–O bond length and Mn–O––Mn bond angle of the parent phase in LSMO–Mo is more significant compared to the other composite materials. This alteration in the crystal structure of LSMO–Mo supports the previously stated hypothesis that some Sr ions detach from the parent perovskite structure and react with Mo to form the (La,Sr)MoO4 phase in the LSMO–Mo composite material.
The electron bandwidth (W) is an important parameter that affects the electronic and magnetic properties of manganites. It can be calculated based on the Mn–O bond length and Mn–O–Mn bond angle obtained from XRD analysis of the sample. The W of manganite materials is affected by several factors, such as crystal structure, chemical doping, and the presence of defects or impurities. A large W generally leads to more metallic behavior and higher mobility of charge carriers, while a small W is associated with more insulating behavior and low mobility of charge carriers [37,38,39]. Moreover, the W significantly affects the magnetic properties of manganite materials. For instance, materials with a small W can exhibit more complex magnetic ordering due to the strong coupling between electron and magnetic degrees of freedom. This complex magnetic ordering can result in interesting phenomena, such as spin glass behavior or magnetic frustration [40, 41]. The W of the samples was calculated by utilizing data obtained from XRD refinement with the aid of the following equation;
where \({\theta }_{Mn-O-Mn}\) is the Mn–O–Mn bond angle, whereas \({d}_{Mn-O}\) represents the Mn–O bond length. Table 1 shows that the LSMO–Mo composite material with a smaller W than the other three indicates a more compact or compressed crystal structure, as confirmed by XRD refinement. The low value of W also suggests weaker electron hopping interactions in the LSMO–Mo composite compared to the other materials. The significant reduction in W calculated for LSMO–Mo composite further indicates a lower probability of electron transition in octahedra, resulting in antiferromagnetic super–exchange interaction being more dominant than ferromagnetic double–exchange interaction, compared to other samples [22].
3.2 Scanning electron microscopy (SEM) analysis
The XRD analysis shows the formation of secondary phases in the perovskite structure when certain transition metals were mixed with the LSMO manganite. SEM analyses were performed to support these findings and verify the presence of these phases in the surface morphologies of the samples. The SEM images of the LSMO, LSMO–Mn, LSMO–Ni, and LSMO–Mo are given in Fig. 3a–d. It is observed that the surface of the LSMO sample has a homogeneous structure, consisting of multi-sided small grains, clusters formed by the occasional merging of these grains, and pores between these grain formations.
It is seen that the surface morphology of LSMO–Mn obtained by mixing Mn with LSMO consists of two different structures (Fig. 3b). In addition to the LSMO perovskite phase, which grows layer by layer with tightly stacked plates beneath the surface, there is a presence of a secondary phase that forms alone and/or occasionally accumulates in clusters on top of the LSMO perovskite phase. EDS analysis was conducted to identify this secondary phase (Fig. 4a), and the results confirmed the presence of the Mn3O4 phase, previously observed within the structure of the LSMO–Mn compound in XRD analysis. There could be several reasons why the two phases have different surface morphologies despite containing the same elements. The presence of the additional Mn element may have contributed to the formation of secondary phases, as previously identified through XRD analysis, which can influence the surface morphology. Another possibility is that grinding itself may have introduced defects or altered the microstructure of the sample, leading to a different surface morphology. Crystallization kinetics during the sintering period can also play a role in the surface morphology of the samples [42]. The rate of nucleation, growth, and coarsening of crystals can affect the microstructure and surface morphology of the samples. Differences in the crystallization kinetics between the two phases may have contributed to the observed differences in surface morphology. The surface structure of the LSMO–Ni composite (Fig. 3c) is very similar to that of LSMO, except for the grain sizes. However, despite the presence of NiO impurities detected in the XRD, there is no visible grain formation associated with these impurities, although grain boundaries are energetically favorable locations for impurities. The XRD analysis reveals that the crystallite sizes of LSMO and NiO in the LSMO–Ni are nearly identical, and there is no discernible variation in grain formation on the sample surface. These findings indicate that the NiO impurity is uniformly dispersed within the LSMO matrix. The SEM image of the LSMO–Mo composite shown in Fig. 3d is presented at a smaller scale compared to others due to the larger grain sizes on its surface. Three distinct formations can be observed on the surface of this composite. On the top surface, there is a densely packed grain formation with clear grain boundaries with grain sizes larger than 2 μm, identified through EDS analysis to belong to the (La,Sr)MoO4 phase, as depicted in Fig. 4b. Additionally, underneath the porosities observed between these grains, there appears to be a different grain formation associated with the LSMO perovskite manganite (gray regions). Furthermore, there is a third formation present on some of the (La,Sr)MoO4 grains, believed to be the Mn3O4 impurity. Therefore, the presence of these three distinct formations in the SEM image of the LSMO–Mo composite is compatible with the existence of three different crystal structures found in the XRD analysis of this composite. The formation of the coarse-grained (La,Sr)MoO4 phase on the smaller LSMO grains can be attributed to several factors. One possibility is the difference in crystal growth rates between the two phases. During the sintering process, if the (La,Sr)MoO4 phase has a higher growth rate compared to LSMO, it can result in the formation of larger grains. Another factor could be the presence of preferential nucleation sites for the (La,Sr)MoO4 phase on the surface of the LSMO grains. These nucleation sites may provide favorable conditions for the formation of larger grains of (La,Sr)MoO4. And, variations in the chemical composition or environmental conditions during the sintering process can also influence the grain size distribution.
3.3 Magnetic properties
The LSMO manganite exhibits a Curie transition temperature (TC) higher than room temperature [29, 43]. The LSMO phase, which is studied as the main phase in this study, exhibits a ferromagnetic to paramagnetic phase transition at approximately 368.2 K (Fig. 5a) [29]. The main goal of this study is to achieve optimized TC near room temperature by intentionally creating secondary phases through the addition of equal amounts of Mn, Ni, and Mo elements to the LSMO manganite. TC corresponds to the temperature at which a significant increase in magnetization occurs, indicating a phase transition from the paramagnetic to the ferromagnetic. Figure 5a–d depict the temperature–dependent magnetization under ZFC and FC conditions for LSMO compound, as well as LSMO–Mn, LSMO–Ni, and LSMO–Mo composite materials. From the M(T) curves of the samples, TC found for LSMO–Mn, LSMO–Ni, and LSMO–Mo composites are approximately 369.4, 304.7, and 277.9 K, respectively. When the M(T) plots of the samples and the TC calculated from these plots are examined, it is seen that LSMO–Ni (around room temperature) and LSMO–Mo composites exhibit significant decreases in both TC and magnetization values. Initial findings regarding the TC of the samples revealed that it is important to investigate the effect of the deliberate creation of secondary phases in manganites to optimize or improve the magnetic and/or magnetocaloric properties. A significant decrease was observed in the magnetization of the manganites containing the secondary phase. There may be some mechanisms that cause these declines. One of these is the presence of secondary phases that may dilute the magnetic moments and disrupt the ordered arrangement of magnetic ions in the sample, leading to a decrease in magnetization [44]. The other is that secondary phases may introduce structural distortions or defects due to possible stress and strain on a lattice of the manganite sample. These distortions may reduce the magnetization by disrupting the alignment of magnetic moments [45]. The interaction between the manganite phase and secondary phases can result in competing magnetic interactions. This can interfere with the cooperative alignment of magnetic moments, thereby reducing the magnetization [46]. Finally, secondary phases may contain impurities or non-magnetic elements that do not contribute to the magnetic properties of the sample. These impurities can dilute the magnetic behavior and lead to a decrease in magnetization.
While the TC of the LSMO–Mn composite remained almost unchanged, the magnetization decreased and a second phase transition was observed originating from the Mn3O4 phase at approximately 42.3 K, where the magnetic ordering or arrangement of atoms within the material changes. The existence of the Mn3O4 phase, which was observed in XRD and SEM analyses, was also seen in the M(T) curve. The Mn3O4 phase is a mixed–valence manganese oxide with interesting magnetic properties. It can exhibit complex magnetic behavior, including magnetic phase transitions and rotating glass–like behavior, depending on factors such as temperature, grain size, and structural defects [47]. The presence of Mn3O4 as the second phase in the LSMO–Mn composite has no significant effect on the general magnetic behavior of the LSMO–Mn composite since Mn3O4 phases exhibit antiferromagnetic behavior at low temperatures. The LSMO–Ni composite exhibits magnetic phase transition around room temperature (~305 K). This result shows that forming a secondary phase willingly in a manganite with a TC above room temperature can optimize the TC of the new sample around room temperature. It is seen that the presence of the NiO impurity phase leads to a decrease in both TC and magnetization. There can be several factors that cause these decreases. NiO is a material that exhibits antiferromagnetic properties [48, 49]. The presence of the impurity phase NiO reduces the ferromagnetic characteristics of the LSMO phase; the magnetic moments of these two phases may not align completely, leading to a decrease in the magnetization and a subsequent decrease in the TC. Finally, the formation of the NiO phase may cause structural distortion in the LSMO lattice (see XRD analyses), since NiO impurity crystallites disappear within the LSMO grains, as revealed in the SEM analysis. The impurity in LSMO grains may introduce stress and/or strain on the lattice of LSMO, leading to a change in the bond angle and bond length of the MnO6 octahedra. This distortion can disrupt the ferromagnetic double exchange interactions and reduce the ferromagnetic interactions, resulting in a decrease in TC and magnetization [50]. As explained in XRD and SEM analyses, the LSMO–Mo composite, consisting of three different phases, exhibited TC below room temperature (~278 K). The decrease in TC compared to LSMO’s can be attributed to the incorporation of (La,Sr)MoO4 phase into the composite. Both the LSMO and (La,Sr)MoO4 phases have different magnetic properties, which can affect the magnetic behavior. LSMO is a ferromagnetic material, meaning it exhibits spontaneous magnetization and aligns its magnetic moments in the same direction below the TC. (La,Sr)MoO4 displays intriguing magnetic behavior, including both paramagnetic and antiferromagnetic properties [51]. At elevated temperatures, (La,Sr)MoO4 typically exhibits paramagnetic characteristics, where the magnetic moments within the material are randomly oriented, leading to a lack of long-range magnetic order. In this paramagnetic state, the material exhibits a weak attraction toward an external magnetic field. As the temperature decreases, (La,Sr)MoO4 has the potential to undergo an antiferromagnetic phase. On the other hand, (La,Sr)MoO4 typically lacks a permanent magnetic moment and does not exhibit magnetic ordering at low temperatures [51]. The (La,Sr)MoO4 in LSMO–Mo may have a dilution effect on the ferromagnetic LSMO phase. The presence of non–magnetic or weakly magnetic (La,Sr)MoO4 reduces the magnetic interactions between the LSMO magnetic moments, leading to a decrease in TC of LSMO–Mo. A considerable decrease in TC may be due to the existence of (La,Sr)MoO4 phase that can disrupt the alignment of magnetic moments in the LSMO phase and impede the exchange interactions between LSMO ions, which is responsible for the ferromagnetic behavior. Similar to the LSMO–Mn composite, an additional phase transition in LSMO–Mo was observed at 44.3 K. This transition arises from the presence of the Mn3O4 phase, which was identified through XRD and SEM analyses.
The W of a material plays a crucial role in its magnetic properties, particularly TC. The W value of the LSMO and LSMO–composites has been calculated and given in Table 1. The LSMO manganite and LSMO–Mn composite have the same TC value (369 K), suggesting that the incorporation of Mn into the LSMO structure does not significantly affect the TC. This similarity in TC could indicate a similar strength of exchange interaction between the magnetic ions in these composites. The calculated W value for LSMO and LSMO–Mn is 0.09546 and 0.09524, respectively, and they are almost equal. As in the case of LSMO–Ni, the impurity phase, which is NiO in the form of crystallites, distributed inside the grains has an influence on the Mn–O–Mn bond angle and Mn–O bond length leading to distorted MnO6 octahedra. As a result, the variation in the W value changes the TC value. There are changes in Mn–O bond length and Mn–O–Mn bond angle of LSMO–Ni composite compared to LSMO manganite, and a decrease in W value was detected. According to the results, it can be interpreted that the decrease in the TC value is due to the decrease in the W value. The lowest W value among the studied samples was calculated for the LSMO–Mo composite and this composite has the smallest TC.
The M(H) curve represents the relationship between the applied magnetic field (H) and the resulting magnetization (M) of the sample and provides valuable insights into the magnetic properties of the material at different temperatures, both below and above TC. In this study, M(H) measurements were carried out with particular temperature steps below and above the TC temperature to obtain more in–depth information about the magnetic properties of the samples and to calculate the (–ΔSM). In Fig. 6a–d, isothermal magnetization M(H) plots of LSMO, LSMO–Mn, LSMO–Ni, and LSMO–Mo were obtained in a changing magnetic field from 0 to 5 T at certain temperatures below and above the TC. Below the TC, it is seen that the magnetizations of all samples almost reach saturation with the increasing field. This behavior indicates that the samples align their magnetic moments parallel to the applied field, resulting in a net magnetization. Above TC, the magnetization of the sample increases linearly with the magnetic field but does not reach saturation. This behavior suggests that thermal energy disrupts the parallel alignment of the magnetic moments and reduces the magnetization. It has been seen that the magnetization of LSMO–Mo decreases compared to the LSMO–Mn, and LSMO–Ni. The decrease in magnetization observed for LSMO–Mo can be attributed to the presence of different impurity phases. LSMO–Mo contains impurities such as Mn3O4, and (La,Sr)MoO4 secondary phase, which are known to disrupt the magnetic ordering and alignment of the magnetic moments within the samples [52]. The impurity phases in LSMO–Mo may possess different magnetic properties. Therefore, these impurities introduce additional magnetic interactions or alter the spin configuration within the samples, resulting in a decrease in magnetization [52]. Additionally, the presence of impurity phases can create defects acting as scattering centers for the magnetic moments and prevent their alignment, causing magnetic disorder within the crystal lattice of the material. This leads to a reduction in the net magnetization of the sample.
Figure 7a–d illustrates the temperature dependence of the (–ΔSM (T)) curves for LSMO, LSMO–Mn, LSMO–Ni, and LSMO–Mo samples under different magnetic field change values. It is widely acknowledged that (\(-\Delta {S}_{M}^{max})\) occurs due to abrupt alterations in magnetization, particularly in proximity to the TC of the sample. It was seen that the peaks representing (\(-\Delta {S}_{M}^{max})\) of all samples occurred at a temperature close to their TC, as expected. In addition, it is observed that these peaks increase as the magnetic field increases. The maximum magnetic entropy change (\(-\Delta {S}_{M}^{max})\) values of all samples for 1 T calculated as 1.50, 1.00, 0.69, and 0.71 J/kg K for LSMO, LSMO–Mn, LSMO–Ni, and LSMO–Mo, respectively. It can be seen that the (\(-\Delta {S}_{M}^{max})\) value of LSMO–Mn, LSMO–Ni, and LSMO–Mo is lower than that of LSMO manganite. It was found that all of the three samples, LSMO–Mn, LSMO–Ni, and LSMO–Mo, contain secondary phases. These secondary phases lead to some important effects resulting in a decrease in (\(-\Delta {S}_{M}^{max})\) of LSMO–Mn, LSMO–Ni, and LSMO–Mo. The presence of Mn3O4, NiO, and (La,Sr)MoO4 phases in LSMO–Mn, LSMO–Ni, and LSMO–Mo, respectively, can disrupt the magnetic ordering and alignment of the magnetic moments. These impurity phases introduce additional magnetic interaction or alter the spin configuration within the samples, leading to a decrease in the magnetization. As (–ΔSM) is closely related to changes in the overall magnetization of the samples, this disruption caused by impurities results in reduced (–ΔSM) in the LSMO–Mn, LSMO–Ni, and LSMO–Mo. It is believed that the significant alteration in the \((-\Delta {S}_{M}^{max})\) is attributed to a strong spin–lattice coupling during the magnetic phase transition. This coupling is likely responsible for an additional (\(-\Delta {S}_{M}^{max})\) occurring near TC.
Table 2 shows a comparison of magnetic and magnetocaloric properties of La0.67Sr0.33MnO3–based composite materials with previous studies. When Table 2 is carefully examined, it is observed that in previous studies, despite an increase in the amount of oxide compound additions to the main perovskite compound, significant changes in the TC values of the compounds did not occur, while changes were observed in the (–ΔSM) values. However, in this study, significant changes in the TC and (–ΔSM) values of the composite materials obtained by adding different metallic elements to the LSMO compound at fixed ratios (0.2) have been found. These findings indicate that in similar studies, composite materials obtained by adding metallic elements in variable ratios rather than fixed ratios can be more precisely optimized in terms of magnetic and magnetocaloric properties.
4 Conclusions
In this study, we have aimed to optimize the TC of the LSMO exhibiting phase transition above room temperature to be near room temperature by introducing Mn, Ni, and Mo pure elements separately in specific proportions. The primary motivation behind this research was to create secondary phases within the LSMO matrix, leveraging the addition of these elements. XRD analyses revealed the presence of secondary phases resulting from the addition of Mn, Ni, and Mo to the LSMO matrix. The existence of secondary phases caused differences in the crystal structures and magnetic properties. These findings provide insights into the impact of incorporating different elements into the LSMO system on its structural characteristics. The SEM analysis provided evidence for the formation of secondary phases in the LSMO–Mn, LSMO–Ni, and LSMO–Mo composites. The surface morphologies exhibited distinct characteristics associated with the presence of these secondary phases. In the case of LSMO–Mn, the surface morphology revealed the coexistence of the perovskite phase and a Mn–based secondary phase, indicating the partial substitution of Mn in LSMO. The LSMO–Ni sample displayed a surface structure similar to LSMO, suggesting a uniform distribution of the NiO impurity phase within the LSMO matrix. The SEM image of the LSMO–Mo composite exhibited three distinct formations corresponding to the (La,Sr)MoO4 phase, the LSMO, and the Mn3O4 impurity phase, consistent with the crystal structures identified in the XRD analysis. The variations in surface morphology can be attributed to factors such as crystal growth rates, preferential nucleation sites, and variations in chemical composition or environmental conditions during the sintering process. The presence of secondary phases significantly influenced the magnetic properties of the samples. The formation of secondary phases in the structure caused a decrease in the TC and magnetization values of the samples. The presence of secondary phases in the LSMO–Mn, LSMO–Ni, and LSMO–Mo may have resulted in the dilution of magnetic moments, disturbances in the lattice structure, and the emergence of competing magnetic interactions. As a result, a decrease in the (\(-\Delta {S}_{M}^{max})\) value was observed. Another important factor to consider is the influence of the electron bandwidth on the magnetocaloric properties. The narrow W observed in the studied samples can lead to a more complex and intricate magnetic ordering, potentially limiting the enhancement of the magnetocaloric effect. Among the samples studied in this work, the sample with the lowest calculated W value has lower TC and (\(-\Delta {S}_{M}^{max})\) values than the others. Finally, a notable achievement of this study is the successful optimization of the TC near room temperature, which has significant implications for practical applications.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
V.K. Pecharsky, K.A. Gschneidner, Giant magnetocaloric effect in Gd5(Si2Ge2). Phys. Rev. Lett. 78, 4494 (1997)
V.K. Pecharsky, K.A. Gschneidner, Magnetocaloric effect and magnetic refrigeration. J. M. Magn. Mater. 200(1–3), 44–56 (1999)
V.K. Pecharsky, K.A. Gschneidner, Magnetocaloric effect from indirect measurements: magnetization and heat capacity. J. Appl. Phys. 86, 565–575 (1999)
X.X. Zhang, J. Tejada, Magnetocaloric effect in La0.67Ca0.33MnOδ and La0.60Y0.07Ca0.33MnOδ bulk materials. Appl. Phys. Lett. 69(23), 3596–3598 (1996)
J.J. Hamilton, E.L. Keatley, H.L. Ju, A.K. Raychaudhuri, V.N. Smolyaninova, R.L. Greene, Low–temperature specific heat of La0.67Ba0.33MnO3 and La0.8Ca0.2MnO3. Phys. Rev. B 54, 14926 (1996)
W. Chen, W. Zhong, D.L. Hou, Y.W. Du, Research on magnetocaloric effect of La1−xKxMnO3. J. Hebei Univ. Sci. Technol. 19(1), 13–16 (1998)
Z.B. Guo, Y.W. Du, J.S. Zhu, H. Huang, W.P. Ding, D. Feng, Large magnetic entropy change in perovskite-type manganese oxides. Phys. Rev. Lett. 78, 1142 (1997)
X. Bohigas, J. Tejada, M.L. Marmnez-Sarrion, S. Tripp, R. Black, Magnetic and calorimetric measurements on the magnetocaloric effect in La0.6Ca0.4MnO3. J. Magn. Magn. Mater. 208, 85–92 (2000)
W. Zhong, W. Chen, C.T. Au, Y.W. Du, Dependence of the magnetocaloric effect on oxygen stoichiometry in polycrystalline La2/3Ba1/3MnO3–d. J. Magn. Magn. Mater. 261, 238–243 (2003)
D.T. Hanh, M.S. Islam, F.A. Khan, D.L. Minh, N. Chau, Large magnetocaloric effect around room temperature in La0.7Ca0.3−xPbxMnO3 perovskites. J. Magn. Magn. Mater. 310(2–3), 2826–2828 (2007)
P.G. Radaelli, D.E. Cox, M. Marezio, S.W. Cheong, P.E. Schiffer, A.P. Ramirez, Simultaneous structural, magnetic, and electronic transitions in La1-xCaxMnO3 with x=0.25 and 0.50. Phys. Rev. Lett. 75(24), 4488–4491 (1995)
A.M. Aliev, A.G. Gamzatov, A.B. Batdalov, A.S. Mankevich, I.E. Korsakov, Structure and magnetocaloric properties of La1−xKxMnO3 manganites. Physica B 406(4), 885–889 (2011)
A.G. Gamzatov, A.B. Batdalov, The relation between magnetoresistance and magnetocaloric effect in La0.85Ag0.15MnO3 manganite. Phys. B: Condens. Matter. 406(10), 1902–1905 (2011)
S. Mnefgui, A. Dhahri, N. Dhahri, K. Hlil, J. Dhahri, The effect deficient of strontium on structural, magnetic and magnetocaloric properties of La0.57Nd0.1Sr0.33−xMnO3 (x=0.1 and 0.15) manganite. J. Magn. Magn. Mater. 340, 91–96 (2013)
M. Smari, I. Walha, E. Dhahri, E.K. Hlil, Structural, magnetic and magnetocaloric properties of Ag-doped La0.5Ca0.5−xAgxMnO3 compounds with 0⩽x⩽04. J. Alloys Compd. 579, 564–571 (2013)
M. Khlifi, M. Bejar, O. EL Sadek, E. Dhahri, M.A. Ahmed, E.K. Hlil, Structural, magnetic and magnetocaloric properties of the lanthanum deficient in La0.8Ca0.2−x-xMnO3 (x=0–0.20) manganites oxides. J. Alloys Compd. 509(27), 7410–7415 (2011)
A.G. Gamzatov, A.B. Batdalov, N.Z. Abdulkadirova, A.M. Aliev, V.V. Khovaylo, T.D. Thanh, N.T. Dung, S.-C. Yu, Giant magnetothermal anomalies and direct measurements of the magnetocaloric effect in Pr0.7Sr0.3−xBaxMnO3 manganites. J. Alloys Compd. 964, 171330 (2023)
Ah. Dhahri, M. Jemmali, E. Dhahri, M.A. Valente, Structural characterization, magnetic, magnetocaloric properties and phenomenological model in manganite La0.75Sr0.1Ca0.15 MnO3 compound. J. Alloys Compd. 638, 221–227 (2015)
A. Jerbi, A. Krichene, N. Chniba-Boudjada, W. Boujelben, Magnetic and magnetocaloric study of manganite compounds Pr0.5A0.05Sr0.45MnO3 (A=Na and K) and composite. Phys. B: Condens. Matter. 477, 75–82 (2015)
M. Pękała, K. Pękała, V. Drozd, J.-F. Fagnard, P. Vanderbemden, Effect of nanocrystalline structure on magnetocaloric effect in manganite composites (1/3)La0.7Ca0.3MnO3/(2/3)La0.8Sr0.2MnO3. J. Alloys Compd. 629, 98–104 (2015)
A. Coşkun, A.E. Irmak, B. Altan, Y.S. Ak, A.T. Coşkun, Tuning the magnetic and magnetocaloric properties of a compound via mixing (1–x).La0.67Ca0.33MnO3+xLa0.67Sr0.33MnO3 (x = 0, 025, 050, 075, 1): composite materials or composite compounds? J. Magn. Magn. Mater. 584, 171104 (2023)
O. Chdil, M. Balli, N. Brahiti, R. Essehli, P. de Rango, P. Fournier, S. Naamane, K. El Maalam, O. Mounkachi, Depicting the roles of CuO secondary phase and heat treatment in driving the magnetic and magnetocaloric features of Pr2∕3Sr1∕3MnO3 manganite. J. Alloy. Compd. 925, 166639 (2022)
A. El Boukili, O. Mounkachi, M. Hamedoun, P. Lachkar, E.K. Hlil, A. Benyoussef, M. Balli, H. Ez-Zahraouy, A study of structural, magnetic and magnetocaloric properties of (1–x)La0.6Ca0.4MnO3/xMn2O3 composite materials. J. Alloys Compd. 859, 158392 (2021)
A.E. Mohamed, V. Vega, M. Ipatov, A.M. Ahmed, B. Hernando, Magnetoresistive and magnetocaloric response of manganite/insulator system. J. Alloys Compd. 657, 495–505 (2016)
A. Rostamnejadi, M. Venkatesan, P. Kameli, H. Salamati, J.M.D. Coey, Magnetocaloric effect in La0.67Sr0.33MnO3 manganite above room temperature. J. Magn. Magn. Mater. 323(16), 2214–2218 (2011)
K. Das, R.R. Chowdhury, S. Midda, P. Sen, I. Das, Magnetocaloric effect study of Pr0.67Ca0.33MnO3-La0.67Sr0.33MnO3 nanocomposite. J. Magn. Magn. Mater. 449, 304–307 (2018)
M.S. Anwar, F. Ahmed, R. Danish, B.H. Koo, Impact of Co3O4 phase on the magnetocaloric effect and magnetoresistance in La0.7Sr0.3MnO3/Co3O4 and La0.7Ca0.3MnO3/Co3O4 ceramic composites. Ceram. Int. 41(1), 631–637 (2015)
T.P. Gavrilova, I.F. Gilmutdinov, J.A. Deeva, T.I. Chupakhina, N.M. Lyadov, I.A. Faizrakhmanov, F.O. Milovich, Yu.V. Kabirov, R.M. Eremina, Magnetic and magnetocaloric properties of (1–x)La0.7Sr0.3MnO3/xNaF composites. J. Magn. Magn. Mater. 467, 49–57 (2018)
A.T. Coşkun, Y.S. Ak, N. Güleç, G. Akça, S.K. Çetin, A. Ekicibil, A. Coşkun, A comparative study of magnetic, and magnetocaloric properties of different transition metal–doped La0.67Sr0.33AO3 (A: Mn Co, Cr, and Fe) samples. J. Mater. Sci.: Mater. Electron. 34, 1257 (2023)
D. Hobbs, J. Hafner, D. Spišák, Understanding the complex metallic element Mn. I. Crystalline and noncollinear magnetic structure of α-Mn. Phys. Rev. B 68, 014407 (2003)
A.S. Ganeshraja, S. Thirumurugan, K. Rajkumar, J. Wang, K. Anbalagan, Ferromagnetic nickel(II) imidazole-anatase framework: an enhanced photocatalytic performance. J. Alloy. Compd. 706, 485–494 (2017)
X. Shen, Y. Wang, Z. Diao, X. Liu, The effect of molybdenum on the magnetic properties of the Nd-Fe-Co-B system. J. Appl. Phys. 61(8), 3433–3435 (1987)
E. Taşarkuyu, A. Coşkun, A.E. Irmak, S. Aktürk, G. Ünlü, Y. Samancıoğlu, A. Yücel, C. Sarıkürkçü, S. Aksoy, M. Acet, Effect of high-temperature sintering on the structural and the magnetic properties of La1.4Ca1.6Mn2O7. J. Alloys Compd. 509, 3717–3722 (2011)
T. Roisnel, J. Rodriguez-Carvajal, WinPLOTR: a Windows tool for powder diffraction patterns analysis. Mater. Sci. Forum 378, 118–123 (2001)
A. Ali, Y. Wai Chiang, R.M. Santos, X-ray diffraction techniques for mineral characterization: a review for engineers of the fundamentals, applications, and research directions. Minerals 12, 205–230 (2022)
P. Scardi, M. Leoni, Polydisperse crystalline systems. Acta Crystallogr. A 57, 604–613 (2001)
Z. Jirak, S. Krupička, E. Pollert, Z. Weiss, Magnetic properties of mixed–valence manganites. J. Magn. Magn. Mater. 53(1–2), 153–158 (1985)
J. Rodríguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192(1–2), 55–69 (1993)
Y. Tokura, N. Nagaosa, Orbital physics in transition–metal oxides. Science 288(5465), 462–468 (2000)
K. Bajaj, V. Bagwe, J. Jesudasan, P. Raychaudhuri, Bandwidth control effects in electron doped manganite La0.7−xYxCe0.3MnO3 thin films. Solid State Commun. 138(10–11), 549–552 (2006)
D.P. Kozlenko, V.P. Glazkov, R.A. Sadykov, B.N. Savenko, V.I. Voronin, I.V. Medvedeva, Structural study of pressure-induced magnetic phase transitions in manganites La0.67Ca0.33MnO3 and Pr0.7Ca0.3MnO3. J. Magn. Magn. Mater. 258–259, 290–292 (2003)
Z. Qing, W. Zhou, W. Xia, H. Li, Crystallization kinetics, sintering, microstructure, and properties of low temperature co-fired magnesium aluminum silicate glass–ceramic. J. Non-Cryst. Solids 486, 14–18 (2018)
D.I. Pchelina, V.D. Sedykh, N.I. Chistyakova, V.S. Rusakov, Y.A. Alekhina, A.N. Tselebrovskiy, B. Fraisse, L. Stievano, M.T. Sougrati, The structural and magnetic features of perovskite oxides La1–xSrxMnO3+δ (x=0.05, 0.10, 0.20) depending on the strontium doping content and heat treatment. Ceram. Int. 49(7), 10774–10786 (2023)
A. Gaur, G.D. Varma, Enhanced magnetoresistance in double perovskite Sr2FeMoO6 through SrMoO4 tunneling barriers. Mater. Sci. Eng.: B 143(1–3), 64–69 (2007)
W.F. Brown Jr., Theory of the approach to magnetic saturation. Phys. Rev. 58, 736–743 (1940)
G.R. Hearne, M.P. Pasternak, R.D. Taylor, P. Lacorre, Electronic structure and magnetic properties of LaFeO3 at high pressure. Phys. Rev. B Condens. Matter 51(17), 11495–21150 (1995)
Y. Ichiyanagi, T. Yamada, Y. Kanazawa, T. Uehashi, Magnetic properties of Mn3O4 nanoparticles. AIP Conf. Proc.. 850, 1155–1156 (2006)
G. Srinivasan, M.S. Seehra, Magnetic susceptibilities, their temperature variation, and exchange constants of NiO. Phys. Rev. B 29, 6295–6298 (1984)
M. Tadic, D. Nikolic, M. Panjan, G.R. Blake, Magnetic properties of NiO (nickel oxide) nanoparticles: blocking temperature and neel temperature. J. Alloy. Compd. 647, 1061–1068 (2015)
J.A. Olarte-Torres, M.C. Cifuentes-Arcila, H.A. Suárez-Moreno, Effects of strain on Lα0.67-xPrxCα0.33Mn0.3, LαMn1-xCoxO3 and LαMn1-xNixO3 magnetite. DYNA 86(211), 278–287 (2019)
M. Muralidharan, V. Anbarasu, A. Elayaperumal, K. Sivakumar, Enhanced ferromagnetism in Cr doped SrMoO4 scheelite structured compounds. J. Mater. Sci.: Mater. Electron. 27, 2545–2556 (2016)
S. Yoon, W. Xie, X. Xiao, S. Checchia, M. Coduri, P. Schuetzenduebe, M. Widenmeyer, S.G. Ebbinghaus, B. Balke, A. Weidenkaff, G. Schütz, K. Son, Site-selective substitution and resulting magnetism in arc-melted perovskite ATiO3-δ (A = Ca, Sr, Ba). J. Am. Ceram. Soc. 106, 6379–7140 (2023)
M. Nasri, J. Khelifi, M. Triki, E. Dhahri, E.K. Hlil, Impact of CuO phase on magnetocaloric and magnetotransport properties of La0.6Ca0.4MnO3 ceramic composites. J. Alloys Compd. 678, 427–433 (2016)
Acknowledgements
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Y.Selim Ak: Performed the SEM and XRD analyses; Experimental support. A.Tulga Coşkun: Performed the SEM and XRD analyses; Preparation of samples; Editing. S. Kılıç Çetin: Proofread the paper; Performed the magnetic measurements. G. Akça: Experimental support; Proofread the paper. A.E. Irmak: Preparation of samples; Visualization; Review & editing. A. Ekicibil: Experimental resources; Proofread the paper; Review & editing; Validation. A. Coşkun: Investigations; Conceptualization; Writing–Review & Editing; Validation; Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ak, Y.S., Coşkun, A.T., Çetin, S.K. et al. Optimizing the curie temperature of La0.67Sr0.33MnO3–based composite materials around room temperature through the addition of Mo, Mn, and Ni metallic elements. J Mater Sci: Mater Electron 35, 1069 (2024). https://doi.org/10.1007/s10854-024-12753-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12753-8