Abstract
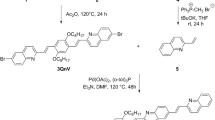
The synthesis of 9–cyano[7]helicene achieved a 62% overall yield with high purity, following a two-step procedure. Characterization was carried out using 1H, 13C, and COSY NMR spectroscopy, as well as FT–IR analysis. The racemic mixture of helicene was effectively separated into P– and M–enantiomers with exceptional optical purity (> 99% ee). This separation allowed us to showcase substantial optical rotations (+ 4800 for the P–enantiomer at λ = 589 nm) and notable electronic circular dichroism (ECD) signals. The organic material exhibited a robust UV absorption band and emitted a vibrant blue light, resulting in a fluorescence quantum yield of 11%. Experimentally determined electronic energy levels revealed HOMO energy of − 5.86 eV and LUMO energy of − 3.25 eV, yielding an electrochemical band gap of 2.61 eV. Furthermore, an in-depth analysis of absorption and ECD spectra, along with molecular electrostatic potential (MEP) and reduced density gradient (RDG) calculations using quantum chemistry, highlighted the material’s fundamental characteristics. These findings collectively suggest that this compound holds promise as a potential candidate for use in electroluminescent devices and OLED technology.













Similar content being viewed by others
Data availability
Data included in article/supplementary material/referenced in article.
References
R.H. Martin, The helicenes. Angew. Chem. Int. Ed. Engl. 13, 649–660 (1974)
Y. Shen, C.F. Chen, Helicenes: synthesis and applications. Chem. Rev. 112, 1463–1535 (2012)
M. Gingras, One hundred years of helicene chemistry. Part 1: non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 42, 968–1006 (2013)
M. Gingras, G. Felix, R. Peresutti, One hundred years of helicene chemistry. Part 2: stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 42, 1007–1050 (2013)
D.B. Amabilino, in Chirality at the nanoscale, nanoparticles, surfaces, materials and more. (Wiley-VCH, Hoboken, 2009), pp.1–418
N. Harada, K. Nakanishi, N. Berova, Electronic CD exciton chirality method: principles and applications. Compr. Chiroptical Spectrosc. 2, 115–166 (2012)
M. Cei, L. Di Bari, F. Zinna, Circularly polarized luminescence of helicenes: a data–informed insight. Chirality. 35, 192–210 (2023)
Y. Liu, Q. Xu, J. Sun, L. Wang, D. He, M. Wang, C. Yang, Insights for vibronic effects on spectral shapes of electronic circular dichroism and circularly polarized luminescence of aza– 7]helicene. Spectrochim. Acta Part. A 239, 118475 (2020)
T. Mori, Chiroptical properties of symmetric double, triple. And multiple helicenes. Chem. Rev. 121, 2373–2412 (2021)
M. Gingras, One hundred years of helicene chemistry. Part 3: applications and properties ofcarbohelicenes. Chem. Soc. Rev. 42(1051), 1051–1095 (2013)
K. Dhbaibi, L. Favereau, J. Crassous, Enantioenriched helicenes and helicenoids containing main-group elements (B, Si, N, P). Chem. Rev. 119, 8846–8953 (2019)
G.M. Upadhyay, A.V. Bedekar, Synthesis and photophysical properties of bi-aza[5] helicene and bi-aza[6]helicene. Tetrahedron. 71, 5644–5649 (2015)
G.M. Upadhyay, H.R. Talele, A.V. Bedekar, Synthesis and photophysical properties of aza[n]helicenes. J. Org. Chem. 81, 7751–7759 (2016)
M. Li, H.-Y. Lu, C.-F. Chen, [5]Helicene derivatives containing aromatic imide moiety: synthesis, structure, and photophysical properties. J. Photochem. Photobiol. A 355, 408–413 (2018)
K. Yamamoto, T. Shimizu, K. Igawa, K. Tomooka, G. Hirai, H. Suemune, K. Usui, Rational design and synthesis of [5]helicene-derived phosphine ligands and their application in Pd-catalyzed asymmetric reactions. Sci. Rep. 6, 36211 (2016)
D. Sakamoto, I.G. Sánchez, J. Rybáček, J. Vacek, L. Bednárová, M. Pazderková, R. Pohl, I. Císařová, I.G. Stará, I. Starý, Cycloiridated helicenes as chiral catalysts in the asymmetric transfer hydrogenation of imines. ACS Catal. 12(17), 10793–10800 (2022)
C.-T. Chen, C.-C. Tsai, P.-K. Tsou, G.-T. Huang, C.-H. Yu, Enantiodivergent Steglich rearrangement of O-carboxylazlactones catalyzed by a chirality switchable helicene containing a 4-aminopyridine unit. Chem. Sci. 8, 524–529 (2017)
E. Anger, M. Srebro, N. Vanthuyne, L. Toupet, S. Rigaut, C. Roussel, J. Autschbach, J. Crassous, R. Réau, Ruthenium-vinylhelicenes: remote metal-based enhancement and redox switching of the chiroptical properties of a helicene core. J. Am. Chem. Soc. 134(38), 15628–15631 (2012)
H. Isla, J. Crassous, Helicene–based chiroptical switches. C R Chim. 19, 39–49 (2015)
M. Shigeno, Y. Kushida, M. Yamaguchi, Molecular switching involving metastable states: molecular thermal hysteresis and sensing of environmental changes by chiral helicene oligomeric foldamers. Chem. Commun. 52, 4955–4970 (2016)
M. Kos, R. Rodríguez, J. Storch, J. Sýkora, E. Caytan, M. Cordier, I. Císařová, N. Vanthuyne, J.A.G. Williams, J. Žádný, V. Církva, J. Crassous, Enantioenriched ruthenium-tris-bipyridine complexes bearing one helical bipyridine ligand: Access to fused multihelicenic systems and chiroptical redox switches. Inorg. Chem. 60(16), 11838–11851 (2021)
S. Oda, B. Kawakami, Y. Yamasaki, R. Matsumoto, M. Yoshioka, D. Fukushima, S. Nakatsuka, T. Hatakeyama, One-shot synthesis of expanded heterohelicene exhibiting narrowband thermally activated delayed fluorescence. J. Am. Chem. Soc. 144, 1 (2022)
J.R. Brandt, X. Wang, Y. Yang, A.J. Campbell, M.J. Fuchter, Circularly polarized phosphorescent electroluminescence with a high dissymmetry factor from PHOLEDs based on a platinahelicene. J. Am. Chem. Soc. 138(31), 9743–9746 (2016)
W. Hua, Z. Liu, L. Duan, G. Dong, Y. Qiu, B. Zhang, D. Cui, X. Tao, N. Cheng, Y. Liu, Deep-blue electroluminescence from nondoped and doped organic light-emitting diodes (OLEDs) based on a new monoaza[6]helicene. RSC Adv. 5, 75–84 (2015)
T. Chen, B. Zhang, Z. Liu, L. Duan, G. Dong, Y. Feng, X. Luo, D. Cui, Synthesis and properties of a thiophene-substituted diaza[7]helicene for application as a blue emitter in organic light-emitting diodes. Tetrahedron Lett. 58, 531–535 (2017)
U.S. Raikar, V.B. Tangod, S.R. Mannopantar, B.M. Mastiholi, Ground and excited state dipole moments of coumarin 337 laser dye. Opt. Commun. 283, 4289–4292 (2010)
J.R. Mannekutla, B.G. Mulimani, S.R. Inamdar, Solvent effect on absorption and fluorescence spectra of coumarin laser dyes: evaluation of ground and excited state dipole moments. Spectrochim. Acta Part. A 69, 419–426 (2008)
J.-D. Chen, H.-Y. Lu, C.-F. Chen, Synthesis and structures of multifunctionalized helicenes and dehydrohelicenes: an efficient route to construct cyan fluorescent molecules. Chem. Eur. J. 16, 11843–11846 (2010)
X.-Y. Wang, X.-C. Wang, A. Narita, M. Wagner, X.-Y. Cao, X. Feng, K. Muellen, Synthesis, structure, and chiroptical properties of a double [7]heterohelicene. J. Am. Chem. Soc. 138, 12783–12786 (2016)
S.K. Collins, A. Grandbois, M.P. Vachon, J. Coté, Preparation of helicenes through olefin metathesis. Angew Chem. Int. Ed. 45, 2923–2926 (2006)
A. Grandbois, S.K. Collins, Enantioselective synthesis of [7]helicene: dramatic effects of olefin additives and aromatic solvents in asymmetric olefin metathesis. Chem. Eur. J. 14, 9323–9329 (2008)
F.B. Mallory, M.J. Rudolph, S.M. Oh, Photochemistry of stilbenes. 8. Eliminative photocyclization of o-methoxystilbenes. J. Org. Chem. 54(19), 4619–4626 (1989)
N. Hafedh, F. Aloui, V. Dorcet, H. Barhoumi, Helically chiral functionalized [6]helicene: synthesis, optical resolution, and photophysical properties. C R Chim. 21, 652–658 (2018)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, Gaussian 09, Revision D, vol. 01 (Gaussian Inc, Wallingford, 2009)
A. Becke, Density functional exchange-energy approximation with correct 7 asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1968)
R. Krishnan, J.S. Binkley, R. Seeger, J.A. Pople, Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980)
M. Ben Braiek, F. Aloui, S. Moussa, B. Ben Hassine, Synthesis and characterization of new helically chiral heptacyclic systems. Tetrahedron Lett. 56, 6580–6584 (2015)
F. Furche, R. Ahlrichs, C. Wachsmann, E. Weber, A. Sobanski, F. Vögtle, S. Grimme, Circular dichroism of helicenes investigated by time-dependent density functional theory. J. Am. Chem. Soc. 122, 1717–1724 (2000)
J. Coates, Interpretation of Infrared spectra, a practical approach, in Encyclopedia of analytical chemistry. ed. by R.A. Meyers (Wiley, Hoboken, 2020), pp.1–23
R.H. Martin, M.J. Marchant, Resolution and optical properties ([α]Max, ORD and CD) of hepta-, octa- and nonahelicene. Tetrahedron. 3, 343–345 (1974)
A. Bossi, L. Falciola, C. Graiff, S. Maiorana, C. Rigamonti, A. Tiripicchio, E. Licandro, P.R. Mussini, Electrochemical activity of thiahelicenes: structure effects and electrooligomerization ability. Electrochim. Acta. 54, 5083–5097 (2009)
H. Kubo, T. Hirose, T. Nakashima, T. Kawai, J.-. Hasegawa, K. Matsuda, Tuning transition electric and magnetic dipole moments: [7]helicenes showing intense circularly polarized luminescence. J. Phys. Chem. Lett. 12, 686–695 (2021)
J.B. Birks, D.J.S. Birch, E. Cordemans, E.V. Donckt, Fluorescence of the higher helicenes. Chem. Phys. Lett. 43, 33–36 (1976)
E.V. Donckt, J. Nasielski, J.R. Greenleaf, J.B. Birks, Fluorescence of the helicenes. Chem. Phys. Lett. 2, 409–410 (1968)
K. Yavari, W. Delaunay, N. De Rycke, T. Reynaldo, P. Aillard, M. Srebro-Hooper, V.Y. Chang, G. Muller, D. Tondelier, B. Geffroy, A. Voituriez, A. Marinetti, M. Hissle, J. Crassous, Phosphahelicenes: from chiroptical and photophysical properties to OLED applications. Chem. Eur. J. 25, 5303–5310 (2019)
A. Ekbote, S.H. Han, T. Jadhav, S.M. Mobin, J. Yeob Lee, R. Misra, Stimuli re-sponsive AIE active positional isomers of phenanthroimidazole as non-doped emitters in OLEDs. J. Mater. Chem. C 6, 2077–2087 (2018)
S. Jhulki, A.K. Mishra, A. Ghosh, T.J. Chow, J.N. Moorthy, Deep blue-emissive bifunctional (hole-transporting + emissive) materials with CIEy ~ 0.06 based on a ‘U’-shaped phenanthrene scaffold f or application in organic light-emitting diodes. J. Mater. Chem. C 4, 9310–9315 (2016)
M. Pecul, K. Ruud, The ab initio calculation of optical rotation and electronic circular dichroism. Adv. Quant. Chem. 50, 185–212 (2005)
M. Spassova, I. Asselberghs, T. Verbiest, K. Clays, E. Botek, B. Champagne, Theoretical investigation on bridged triarylamine helicenes: UV/visible and circular dichroism spectra. Chem. Phys. Lett. 439, 213–218 (2007)
Y. Nakai, T. Mori, Y. Inoue, Theoretical and experimental studies on circular dichroism of carbo[n]helicenes. J. Phys. Chem. A 116, 7372–7385 (2012)
J. Pommerehne, H. Vestweber, W. Guss, R.F. Mahrt, H. Bassler, M. Porsch, J. Daub, Efficient two layer leds on a polymer blend basis. Adv. Mater. 7, 551–554 (1995)
J.L. Bredas, R. Silbey, D.S. Boudreaux, R.R. Chance, Chain-length dependence of electronic and electrochemical properties of conjugated systems: polyacetylene, polyphenylene, polythiophene, and polypyrrole. J. Am. Chem. Soc. 105, 6555–6559 (1983)
L. Shi, Z. Liu, G. Dong, L. Duan, Y. Qiu, J. Jia, W. Guo, D. Zhao, D. Cui, X. Tao, Synthesis, structure, Properties, and application of a carbazole-based diaza[7]helicene in a deep-blue-emitting OLED. Chem. Eur. J. 18, 8092–8099 (2012)
J.S. Murray, P. Politzer, The electrostatic potential: an overview. WIREs Comp. Mol. Sci. 1, 153–163 (2011)
B.L. Chittari, S.P. Tewari, Theoretical studies on aminoborane oligomers. Comp. Theor. Chem. 1020, 151–156 (2013)
T. Lu, F. Chen, Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012)
W. Humphrey, A. Dalke, K. Schulten, Visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996)
Acknowledgements
The authors are grateful to the DGRS (Direction Générale de la Recherche Scientifique) of the Tunisian Ministry of Higher Education and Scientific Research for financial support. The authors thank Dr. Nicolas Vanthuyne (Aix-Marseille Université, Service 432-Plateforme de chromatographie chirale ISM2-UMR7313) for HPLC facilities.
Funding
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Contributions
Ibtissem Hajji: performed the experiments; helped in the manuscript preparation. Mourad Chemek: performed the theoretical calculations. Abdullah Y. A. Alzahrani: contributed to experimental characterizations and interpreted the data. Béchir Ben Hassine: contributed to the design of the experiments. Faouzi Aloui: conceived and designed the experiments; contributed to drafting; revised and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hajji, I., Chemek, M., Alzahrani, A.Y.A. et al. Synthesis, photophysical and chiroptical properties of 9–cyano[7]helicene for OLED applications. A combined experimental and theoretical investigation. J Mater Sci: Mater Electron 35, 542 (2024). https://doi.org/10.1007/s10854-024-12275-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12275-3