Abstract
The construction of an efficient bismuth iron oxide shows great potential in excellent crystal structure properties and visible light photocatalysis. However, it is challenging to synthesize nanostructures with the desired morphologies. For the first time, the bismuth iron oxide is fabricated using sol–gel synthesis method, exhibiting the desired crystallite size and tuning the types of heterojunction nanostructures by regulating the concentration of Fe3+ and calcination temperature. As a result, the feeding ratios of bismuth ferrite materials Bi2Fe4O9, BiFeO3/Bi2Fe4O9, BiFeO3, BiFeO3/Bi25FeO40, and Bi25FeO40 are 2:1, 1.5:1, 1:1, 0.75:1, 0.5:1, and 0.04:1, respectively. Besides, the calcination temperature not only influences the granularity of bismuth iron oxide but also promotes the phase transformation from BiFeO3 to Bi2Fe4O9. Moreover, BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterojunction nanostructures display strong interactions between BiFeO3–Bi2Fe4O9 and Bi25FeO40. Besides, BiFeO3/Bi25FeO40 heterojunction nanostructures exhibit obvious grain boundary with the smallest bandgap. This study presents far-reaching implications and provides pathways to prepare BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterojunction nanostructures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bismuth iron oxide, as a typical semiconductor, is widely employed because of its narrow bandgap and excellent storage, sensing, and multiferroic properties [1, 2]. However, further performance optimization of bismuth ferrite is a difficult task because the preparation of heterojunction nanostructures requires complex reaction conditions [3, 4]. Hence, further improving the catalytic performance of bismuth ferrite for photocatalytic reactions by doping other metallic atoms and modifying the morphology of what requires the pursuit of heterojunction nanostructures with a controllable crystal phase [5,6,7]. Moreover, the functions of redox activity, photocatalytic performance, and electron donor–acceptor pair in heterojunction materials are diverse and powerful [8, 9]. Among these several strategies, constructing heterojunction structures can significantly improve the utilization of electron holes, while enhancing the electronic mobility and showing excellent optoelectronic properties [10,11,12].
Numerous research efforts have been devoted to fabricate the name material with a desirable structure and excellent ferroelectric properties. However, the influence of crystal structure on morphology, crystallite size, and performance of bismuth iron oxide is still limited. Furthermore, the structure–property relationship of bismuth iron oxide with a heterojunction nanostructure is relatively complex, whereas main reasons for the precise regulation of phase composition content and grain size of the heterojunction are still unknown. Hence, the phase composition of optimal design and precision synthesis of BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterojunction nanostructures through the feed ratio of Bi, Fe, and calcination temperature by simple Fe3+ doping. These findings present far-reaching implications and provide pathways for the further development of heterojunction nanostructure with metallic doping of bismuth iron oxide.
Based on the aforementioned discussion, we designed and prepared BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterojunction nanostructures by the sol–gel method. Herein, the preparation of heterojunction nanostructures is achieved by adjusting the crystallite size and phase composition of bismuth iron oxide. Furthermore, BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterojunction nanostructures display strong interactions between BiFeO3-Bi2Fe4O9 and Bi25FeO40. Moreover, BiFeO3/Bi25FeO40 heterojunction nanostructures exhibit obvious grain boundaries with a small bandgap. This study presents a simple route to prepare BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 dual heterojunction nanostructures.
2 Experimental section
Bismuth iron oxide powder was prepared by the citric acid-based sol–gel method. Different molar ratios of Bi(NO3)3·5H2O (Tianjin Kermel Chemical Reagent Co. Ltd., China) and Fe(NO3)3·9H2O (Tianjin Tianli Chemical Reagents Co. Ltd., China) were mixed in a beaker under constant stirring to obtain a mixture. Then, 20 mL of ethylene glycol methyl ether (Tianjin Kermel Chemical Reagent Co. Ltd., China) and 20 μL of 0.2-M HNO3 (Tianjin Kermel Chemical Reagent Co. Ltd., China) were added into a three-necked flask and magnetically stirred in a KQ-300 VDE ultrasonic cleaner (Kunming Ultrasonic Instruments Co. Ltd., China) to obtain a clear solution. Then, 0.08 mol of citric acid (Tianjin Zhiyuan Chemical Reagent Co. Ltd., China) and 10 mL of ethylene glycol (Tianjin Kermel Co. Ltd., China) were added under magnetic stirring. Secondly, the reaction vessel was placed in a DF-10IB water bath (Changzhou Nuoji Instruments Co. Ltd., China) at 60 °C for 1 h. The as-obtained dark/brown gel was dried in a drying oven at 60 °C and the dried sample was placed in a muffle furnace after grounding into powder in an agate crucible. Finally, the sample in the muffle furnace was treated at 200 °C for 30 min and, then, heated to a certain temperature for 60 min, followed by cooling to room temperature. The heating and cooling rates were kept at 4.7 °C min−1. The as-prepared sample was taken out and characterized as bismuth ferrite powder.
The crystal structure of bismuth iron oxide was studied by an X-ray diffractometer (Bruker D8 Advance), equipped with a Cu Kα radiation source (λ = 1.5418 Å). The optical performance was measured using UV–vis spectroscopy (UV-2600) and X-ray photoemission spectroscopy (XPS) was performed using a Thermo SCIENTIFIC ESCALAB 250Xi spectrometer. The morphology and microstructure of the as-prepared materials were characterized by a scanning electron microscope (SEM, JSM-7800F) and transmission electron microscopy (TEM, JEOL-JEM 2100 F), equipped with a field emission gun operating at 200 kV.
3 Results and discussion
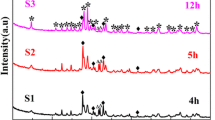
The structural characteristics of bismuth iron oxide were investigated comprehensively. As shown in Fig. 1a, at the calcination temperature of 550 °C, the diffraction peaks of bismuth ferrite oxide (BFO) at 22.49°, 31.80°, and 32.13° can be attributed to (101), (012), and (110) planes of BiFeO3, respectively. When the calcination temperature is less than 550 °C, the peak at 2θ = 27.77° reveals the presence of Bi2O3, indicating that the low temperature is favorable for the precipitation of the impurity phase. When the annealing temperature is greater than 550 °C, the peak at 2θ = 29.72° corresponds to the Bi2Fe4O9 phase. Hence, it can be inferred BiFeO3 is quite unstable and easily transformed into Bi2O3 and Bi2Fe4O9 due to its unstable structure [13]. Therefore, the high temperature is favorable for the precipitation of Bi2Fe4O9 (JCPDS: 25–0090) phase. This confirms that the temperature can control the generation of bismuth iron oxide. The calculation of the relative content is consistent with that in the literature, where (IA is the intensity of the X-ray diffraction peak on the BiFeO3 (101) plane; IR is the intensity of the X-ray diffraction peak on the Bi2Fe4O9 (121), and Bi25FeO40 (310) plane is used for all the following calculations of the relative content. As presented in Fig. 1b and c, when Bi3+/Fe3+ is 1:1.5, different calcination temperatures indicate that the pure BiFeO3 phase is generated at 500 °C in the presence of excess Fe3+ and Bi2Fe4O9 phase is generated and its content gradually increases with increasing temperature, indicating that BiFeO3 phase transforms into BiFeO3/Bi2Fe4O9 composite structure at T > 550 °C. Thus, the pure BiFeO3 phase can be prepared in the presence of excess Fe3+ and at low temperatures [14]. Figure 1d and e shows the influence of different calcination temperatures when Bi3+/Fe3+ is 0.75:1, which is the ratio with minimum bandgap width. The temperature renders little effect on the concentration of BiFeO3/Bi25FeO40 composite phase. Figure 1f and g shows the influence of different stoichiometric ratios at 700 °C. The given temperature is selected because it renders the smallest bandgap width. It can be seen that the characteristic diffraction peaks of BiFeO3 and Bi2Fe4O9 are quite clear when the molar ratio of Bi3+/Fe3+ is 1:1.25 at 700 °C. These peaks clearly indicate the high content of BiFeO3 and low content of Bi2Fe4O9. Also, these peaks appear due to the presence of sufficient oxygen. However, both BiFeO3 and Bi2Fe4O9 possess similar diffraction peaks when the molar ratio of Bi3+/Fe3+ is 1:1.5 and the intensity of the characteristic diffraction peak of Bi2Fe4O9 is obviously enhanced when the molar ratio of Bi3+/Fe3+ is 1:1.75. Moreover, only the characteristic diffraction peaks of pure Bi2Fe4O9 exist and no characteristic diffraction peaks of other secondary phases exist when the molar ratio of Bi3+/Fe3+ is 1:2. In addition, the as-generated pure BiFeO3 and Bi2Fe4O9 phases may correspond to the Bi3+/Fe3+ stoichiometry ratio of 1:1 [15, 16] and 1:2 [17], respectively, whereas excess Fe3+ promotes the transformation of Bi2Fe4O9 from BiFeO3 phase, generating intermediate phases according to the molar ratio. As exhibited in Fig. 1h and i, the influence of different stoichiometric ratios is studied at 700 °C, which is a temperature with the smallest bandgap width. Herein, the content composition of BiFeO3 exhibits a slower decay compared to the Bi25FeO40 (JCPDS: 46-0416) with the decrease of Bi3+/Fe3+ ratio from 1:0.75 to 1:0.04 at 700 °C. Also, different concentrations of BiFeO3/Bi25FeO40 composite phase are formed with the decrease of Fe3+ content and only Bi25FeO40 exists when the molar ratio of Bi3+/Fe3+ is 1:0.04, which corresponds to the Bi3+/Fe3+ stoichiometric ratio of 25:1. Furthermore, what effectively avoids the destruction of BiFeO3 crystal structure at high temperatures.
To explore interactions within BFO, XPS analysis determined the valence states and surface chemistry bonds of constituent elements. In high-resolution Bi 4f spectra, the fitted Bi 4f region shows two peaks near 158.68 eV and 164.11 eV, corresponding to Bi 4f7/2 and Bi 4f5/2, respectively. This implies the existence of Bi3+ valence states in both samples [18]. Moreover, Fe 2p regions split into two peaks for Fe 2p3/2 and Fe 2p1/2, which are fitted using the Gaussian–Lorentzian fitting. Spin–orbit binding energies located near 710 eV and 723.5 eV can be attributed to Fe 2p3/2 and Fe 2p1/2 of Fe2+ species, and the peaks centered near 711 eV and 724.5 eV can be assigned to Fe 2p3/2 and Fe 2p1/2 configuration of Fe3+ species, respectively. Besides, this demonstrates the chemical valence states of Fe cations in Bi25FeO40 and BiFeO3/Bi25FeO40 samples. The peaks located at 713.17 eV and 712.64 eV correspond to Fe4+ [19, 20]. The content of Fe species is shown in Table 1. In the high-resolution O 1 s spectra, two peaks with different binding energies are found, which belong to two different oxygen species. The peak near 529 eV can be attributed to the lattice oxygen (OL), whereas the peak at 530 eV corresponds to the absorbed oxygen species, representing non-lattice oxygen. As shown in Fig. 2a–c, XPS spectra of O 1 s, Bi 4f, and Fe 2p do not exhibit any obvious shift in peak position for BiFeO3 and BiFeO3-Bi2Fe4O9, confirming the presence of weaker interactions between BiFeO3 and Bi2Fe4O9 and indicating that BiFeO3-Bi2Fe4O9 chemical bond interactions are similar to BiFeO3. Furthermore, in the XPS spectra of three materials, the Bi 4f, Fe 2p, and O 1 s peaks of BiFeO3/Bi2Fe4O9 negatively shift around 0.1–0.4 eV as compared to BiFeO3 and Bi2Fe4O9, indicating the presence of strong interactions between BiFeO3 and Bi2Fe4O9. Moreover, the heterojunction nanostructure properties of BiFeO3–Bi2Fe4O9 alloy models and BiFeO3/Bi2Fe4O9 heterojunction nanostructure are evaluated. In Fig. 2d–f, the peaks in high-resolution Bi 4f, Fe 2p, and O 1 s spectra from BiFeO3/Bi25FeO40 heterojunction nanostructure are negatively shifted around 0.1–0.53 eV compared to BiFeO3 and Bi25FeO40, indicating the presence of strong electronic interactions between BiFeO3 and Bi25FeO40, as exhibited in Fig. 2g–i. Moreover, compared to alloy precipitated (BiFeO3–Bi2Fe4O9) by temperature controlled with the heterojunction structure synthesized (BiFeO3/Bi2Fe4O9) by stoichiometric ratio calculation is weaker, the interactions between multiferroic materials (BiFeO3/Bi2Fe4O9) and bismuth-rich materials (BiFeO3/Bi25FeO40) are much stronger than pure bismuth iron oxide phases (BiFeO3, Bi2Fe4O9, and Bi25FeO40).
The morphologies of as-prepared Bi2Fe4O9 and BiFeO3/Bi2Fe4O9 heterojunctions are investigated by SEM, TEM, and HRTEM to confirm the nanosheet-like morphology and heterojunction between BiFeO3 and Bi2Fe4O9. As shown in Fig. 3a–c, Bi2Fe4O9 displayed obvious agglomeration to form highly polymeric channels and exhibited vivid lattice fringes with an interplanar spacing of 0.316 nm, corresponding to the (121) planes. As shown in Fig. 3d, BiFeO3/Bi2Fe4O9 exhibits pores formed due to extensive agglomeration and the surface morphology becomes relatively loose compared to Bi2Fe4O9. As displayed in Fig. 3e and f, the observed angle between two crystallographic planes is consistent with the theoretical value and the lattice spacings of 0.252 nm and 0.316 nm correspond to (202) and (121) planes of BiFeO3 (JCPDS # 25-0090) [21] and Bi2Fe4O9 (JCPDS # 20-0169) [22, 23], respectively. These results are consistent with XRD and XPS results. Moreover, the successful incorporation of Bi2Fe4O9 into BiFeO3/Bi2Fe4O9 confirms that the proposed preparation method realizes the construction of heterojunction nanostructures.
Furthermore, SEM, TEM, and HRTEM images are observed to further analyze the heterojunction and nanosheet morphologies. Figure 4a–c displays loose irregular massive nanosheet-like morphology. Also, the HRTEM image exhibits well-defined lattice fringes with an interplanar spacing of 0.32 nm, corresponding to (310) crystal planes of Bi25FeO40. As shown in Fig. 4d, what exhibits relatively loose and irregular block-like morphology and the surface exhibits obviously different grain boundaries compared to Bi25FeO40. As exhibited in Fig. 4e and f, d(202) = 0.252 may be the lattice spacing of BiFeO3 [21] and another lattice spacing of d(622) = 0.325 may correspond to Bi25FeO40 (JCPDS # 46-0416). Meanwhile, the material is also identified as BiFeO3 and Bi25FeO40 by XRD, as shown in Fig. 1. Elemental maps (Fig. 4g–i) demonstrate the corresponding elemental distribution, which is consistent with the observations, indicating that the grain boundaries of BiFeO3 and Bi25FeO40 are formed. Combined with XPS, it can be inferred that strong interactions exist between BiFeO3 and Bi25FeO40, confirming that a certain interfacial effect exists between BiFeO3 and Bi25FeO40.
a SEM and b TEM images of Bi25FeO40 nanostructures; c HRTEM image of Bi25FeO40 nanostructures; d SEM and e TEM images of BiFeO3/Bi25FeO40 heterojunction nanostructure; f HRTEM image of BiFeO3/Bi25FeO40 heterojunction nanostructures; and g–i EDS elemental maps of Bi, Fe, and O in BiFeO3/Bi25FeO40 heterojunction nanostructures
As shown in Fig. 5, the UV–vis diffuse reflectance absorption spectra exhibit a red shift from the UV region to the visible region. In the visible band, the spectral lines are shifted toward the red end of the spectrum by a certain distance. One should note that bismuth ferrite oxide exhibits light absorption properties around 500–600 nm. The corresponding forbidden bandwidths and optical bandgap of the tested sample are fitted with the Tauc equation based on the relationship between (αhυ)2 and hυ [24]. As shown in Fig. 5a and b, the magnitude of the forbidden bandwidths at 500 °C, 550 °C, 600 °C, 650 °C, and 700 °C is 1.96 eV, 2.05 eV, 1.98 eV, 1.98 eV, and 1.92 eV, respectively. The contents of the forbidden bandwidth are shown in Table 2, illustrating that the energy bandgap of bismuth ferrite oxide nanostructures is changed in the range of 1.92–2.05 eV by controlling the calcination temperature. In addition, the forbidden bandwidth at 500 °C is significantly smaller than at 550 and 650 °C, which is probably due to the generation of Bi2O3/BiFeO3 at 500 °C [25]. As the temperature increases to 700 °C, the forbidden bandwidth decreases and the grains become denser [21]. This proves the conjecture that agglomeration occurs during the high-temperature calcination process. Furthermore, bismuth-rich materials (BiFeO3/Bi25FeO40) are better than multiferroic materials (BiFeO3/Bi2Fe4O9). As shown in Fig. 5d, e, g, and h, the optical bandgap for different molar ratios of Bi3+/Fe3+ (1:2, 1:1.75, 1:1.5, 1:1.25, 1:1, 1:0.75, 1:0.5, 1:0.25, and 1:0.04) is found to be 1.94 eV, 1.90 eV, 1.87 eV, 1.89 eV, 1.92 eV, 1.77 eV, 1.93 eV, 1.99 eV, and 2.12 eV, respectively. The contents of the forbidden bandwidth are shown in Table 3, indicating that the bandgap width of heterojunction nanostructure materials (BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40) is smaller than the pure bismuth iron oxide phase (BiFeO3, Bi2Fe4O9, and Bi25FeO40), which is consistent with XPS results. Moreover, bismuth-rich materials (BiFeO3/Bi25FeO40) are better than multiferroic materials (BiFeO3/Bi2Fe4O9). As exhibited in Fig. 5c, f, and i, the Mott–Schottky curves (representing the values of EVB) of BiFeO3-Bi2Fe4O9 are highly similar to BiFeO3, indicating that the precipitated impurities render a little influence on the structure of bismuth iron oxide, as revealed by XPS results. In addition, ECB can be given as follows:
UV–vis DRS spectra, Kubelka–Munk curves, Mott–Schottky curves, and nanostructured heterostructures of bismuth ferrite oxide materials: a–c at different calcination temperatures with Bi3 + /Fe3 + stoichiometric ratio of 1:1; d–i with different molar ratios of Bi3 + /Fe3 + at the calcination temperature of 700 oC; and j as-formed BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterogeneous nanostructures
Ee refers to the energy of free electrons on the hydrogen scale (∼4.5 eV), X represents the geometric mean of electronegativity, and Eg corresponds to the bandgap of the semiconductor compound. EVB of Bi2Fe4O9, BiFeO3/Bi2Fe4O9, BiFeO3, BiFeO3/Bi25FeO40, and Bi25FeO40 is found to be 1.61 eV, 1.3 eV, 1.42 eV, 1.18 eV, and 1.69 eV, respectively, whereas ECB is found to be − 0.33 eV, − 0.57 eV, − 0.5 eV, − 0.59 eV, and − 0.43 eV, respectively. The contents of the bandgaps are shown in Table 4, indicating that the heterojunction materials are more prone to electron–hole transitions. Figure 5j presents the schematic illustration of the as-formed heterostructure of BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40.
To further compare the intrinsic activity of bismuth ferrite oxide, the bandgap widths and corresponding crystallite sizes of heterojunction nanostructures at different calcination temperatures were calculated. From the diffuse reflectance spectrum, the Kubelka–Munk function can be used to estimate the optical absorption edge energy (Figure b, e). The forbidden bandwidths change significantly when the wavelength is around 600 nm, and the spectral lines of the spectrum move toward the red end and the wavelength becomes longer, indicating a significant red shift phenomenon (Figure a, d). The forbidden bandwidths of BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 at 500 °C, 550 °C, 600 °C, 650 °C, and 700 °C are 1.94/1.91 (eV), 1.99/1.91 (eV), 1.90/1.85 (eV), 1.90/1.84 (eV), and 1.87/1.77 (eV), respectively, as shown in Table 5. The activity of these bismuth ferrite oxide materials is also supported by comparing their bandgap width, while bandgap width of BiFeO3/Bi25FeO40 material is smaller than BiFeO3/Bi2Fe4O9 material. The crystallite size can be generally calculated using Scherrer’s equation, as described elsewhere [26]. After the continuous calcination temperature tests, crystallite size exhibits different variations, where the crystallite size of BiFeO3 and Bi25FeO40 materials exhibits a severe increase and Bi2Fe4O9 crystals exhibits a little change in crystallite size (Fig. 6c, f and Supplementary Fig. 1). This indicates that the temperature promotes the growth of BiFeO3 and Bi25FeO40 grains, and the higher the temperature, the larger the grain size of BiFeO3 and Bi25FeO40 grains. In addition, the different Bi3+/Fe3+ ratio also affects the granularity of BiFeO3, Bi2Fe4O9, and Bi25FeO40 materials as demonstrated in (Supplementary Fig. 2, 3).
4 Conclusion
In conclusion, the desired phase composition, heterojunction nanostructure, and crystallite size of bismuth iron oxide, with an optimal concentration of Fe3+, were successfully prepared by the sol–gel method. The experimental results reveal that BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterojunction nanostructures display strong interactions between BiFeO3-Bi2Fe4O9 and Bi25FeO40. Besides, this bandgap width of BiFeO3/Bi2Fe4O9 and BiFeO3/Bi25FeO40 heterojunction nanostructures is smaller than that of BiFeO3, Bi2Fe4O9 and Bi25FeO40 materials. At the same time, synthesizing heterojunction, increasing calcination temperature, and reducing Fe3+ concentration can precisely control the heterojunction structure, phase composition, grain size, and narrow bandgap of the materials. In addition, BiFeO3/Bi25FeO40 heterojunction has obvious grain boundaries and minimum bandgap. These findings provide pathways for the photocatalytic application of bismuth iron oxide and redox reactions. Meanwhile, the crystallite size plays an important role in controlling the optical, magnetic, electrical, and catalytic properties. Overall, these results have far-reaching implications from the viewpoint of Fe3+ content on the structure and performance of bismuth oxide.
Data availability
Some data can be provided if required.
Code availability
Some data can be provided if required.
References
M. Zhang, M.S. Cao, Q.Q. Wang, X.X. Wang, W.Q. Cao, H.J. Yang, J. Yuan, A multifunctional stealthy material for wireless sensing and active camouflage driven by configurable polarization. J. Mater. Sci. Technol. 132, 42–49 (2023). https://doi.org/10.1016/j.jmst.2022.05.046
R.R. Awasthi, S.K. Trivedi, V.S. Chandel, M. Shariq, H.J. Alathlawi, S.P. Singh, Effect of Zn doping on structural/microstructural, surface topography, and dielectric properties of Bi2Fe4O9 polycrystalline nanomaterials. ACS Omega 8, 15960–15967 (2023). https://doi.org/10.1021/acsomega.2c07604
T. Zhang, Y. Shen, Y. Qiu, Y. Liu, R. Xiong, J. Shi, J. Wei, Facial synthesis and photoreaction mechanism of BiFeO3/Bi2Fe4O9 Heterojunction nanofibers. ACS Sustain. Chem. Eng. 5(6), 4630–4636 (2017). https://doi.org/10.1021/acssuschemeng.6b03138
L. Wu Yt, G.M. Han, Xu. Sm, Lh. Guo, Controllable synthesis and catalytic activity of bismuth silicate: Thin-layered photocatalysts with the effect of template agent. Ceram. Int. 49, 6996–7004 (2023). https://doi.org/10.1016/j.ceramint.2022.10.141
Y. Liu, H. Guo, Y. Zhang, W. Tang, X. Cheng, W. Li, Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: singlet oxygen generation and degradation for aquatic levofloxacin. Chem. Eng. J. 343, 128–137 (2018). https://doi.org/10.1016/j.cej.2018.02.125
A. Kirsch, M.M. Murshed, M. Schowalter, A. Rosenauer, T.M. Gesing, Nanoparticle precursor into polycrystalline Bi2Fe4O9: an evolutionary investigation of structural. Morphol. Opt. Vibrat. Prop. J Phys Chem C 120, 18831–18840 (2016). https://doi.org/10.1021/acs.jpcc.6b04773
A. Kirsch, M.M. Murshed, P. Gaczynski, K.D. Becker, T.M. Gesing, Bi2Fe4O9: structural changes from nano- to micro-crystalline state. Zeitschrift für Naturforschung B 71, 447–455 (2016). https://doi.org/10.1515/znb-2015-0227
E. Zhu, S. Zhao, H. Du, Y. Ma, K. Qi, C. Guo, Z. Su, X. Wang, Z. Wu, Z. Wang, Construction of Bi2Fe4O9 red phosphorus heterojunction for rapid and efficient photo-reduction of Cr(VI). J. Am. Ceram. Soc. 104, 5411–5423 (2021). https://doi.org/10.1111/jace.17782
D. Wang, X. Dong, Y. Lei, C. Lin, D. Huang, X. Yu, X. Zhang, Fabrication of Mn/P co-doped hollow tubular carbon nitride by a one-step hydrothermal–calcination method for the photocatalytic degradation of organic pollutants. Catal. Sci. Technol. 18, 5709–5722 (2022). https://doi.org/10.1039/D2CY01107G
M. Peng, Y. Yu, Z. Wang, X. Fu, Y. Gu, Y. Wang, K. Zhang, Z. Zhang, M. Huang, Z. Cui, F. Zhong, P. Wu, J. Ye, T. Xu, Q. Li, P. Wang, F. Yue, F. Wu, J. Dai, C. Chen, W. Hu, Room-temperature blackbody-sensitive and fast infrared photodetectors based on 2D Tellurium/Graphene Van der Waals heterojunction. ACS Photonics 9, 1775–1782 (2022). https://doi.org/10.1021/acsphotonics.2c00246
X. Li, L. Wu, S. Cheng, C. Chen, P. Lu, First-principles study on optoelectronic properties of Cs2PbX4-PtSe2 van der Waals heterostructures. RSC Adv. 12, 2292–2299 (2022). https://doi.org/10.1039/D1RA08574C
N. Mwankemwa, H.E. Wang, T. Zhu, Q. Fan, F. Zhang, W. Zhang, First principles calculations investigation of optoelectronic properties and photocatalytic CO2 reduction of (MoSi2N4)5-n/(MoSiGeN4)n in-plane heterostructures. Res. Phys. 37, 105549 (2022). https://doi.org/10.1016/j.rinp.2022.105549
M.I. Morozov, N.A. Lomanova, V.V. Gusarov, Specific features of BiFeO3 formation in a mixture of Bismuth(III) and Iron(III) Oxides. Russ. J. Gen. Chem. 73, 1676–1680 (2003). https://doi.org/10.1023/B:RUGC.0000018640.30953.70
J. Wei, Y. Liu, X. Bai, C. Li, Y. Liu, Z. Xu, P. Gemeiner, R. Haumont, I.C. Infante, B. Dkhil, Crystal structure, leakage conduction mechanism evolution and enhanced multiferroic properties in Y-doped BiFeO3 ceramics. Ceram. Int. 42(12), 13395–13403 (2016). https://doi.org/10.1016/j.ceramint.2016.05.106
S.W. Lee, C.S. Kim, Growth of multiferroics BiFeO3 thin films by sol–gel method. J. Magn. Magn. Mater. 304(2), 772–774 (2006). https://doi.org/10.1016/j.jmmm.2006.02.219
J.H. Xu, H. Ke, D.C. Jia, W. Wang, Y. Zhou, Low-temperature synthesis of BiFeO3 nanopowders via a sol–gel method. J. Alloy. Compd. 472, 473–477 (2009). https://doi.org/10.1016/j.jallcom.2008.04.090
P. Psathas, Y. Georgiou, C. Moularas, G.S. Armatas, Y. Deligiannakis, Controlled-phase synthesis of Bi2Fe4O9 & BiFeO3 by flame spray pyrolysis and their evaluation as non-noble metal catalysts for efficient reduction of 4-nitrophenol. Powder Technol. 368, 268–277 (2020). https://doi.org/10.1016/j.powtec.2020.04.059
M. Pooladi, H. Shokrollahi, S.A.N.H. Lavasani, H. Yang, Investigation of the structural, magnetic and dielectric properties of Mn-doped Bi2Fe4O9 produced by reverse chemical co-precipitation. Mater. Chem. Phys. 229, 39–48 (2019). https://doi.org/10.1016/j.matchemphys.2019.02.076
X. Wang, M. Zhang, P. Tian, W.S. Chin, C.M. Zhang, A facile approach to pure-phase Bi2Fe4O9 nanoparticles sensitive to visible light. Appl. Surf. Sci. 321, 144–149 (2014). https://doi.org/10.1016/j.apsusc.2014.09.166
Y. Wu, S. Wang, Y. Gao, X. Yu, H. Jiang, B. Wei, Z. Lü, In situ growth of copper-iron bimetallic nanoparticles in A-site deficient Sr2Fe1.5Mo0.5O6-δ as an active anode material for solid oxide fuel cells. J. Alloys Compd. 926, 166852 (2022). https://doi.org/10.1016/j.jallcom.2022.166852
R. Verma, A. Chauhan, K.M. Neha, R. Batoo, M. Kumar, E.H. Hadhi, Raslan, effect of calcination temperature on structural and morphological properties of bismuth ferrite nanoparticles. Ceram. Int. 47(3), 3680–3691 (2021). https://doi.org/10.1016/j.ceramint.2020.09.220
Y. Xiong, M. Wu, Z. Peng, N. Jiang, Q. Chen, Hydrothermal synthesis and characterization of Bi2Fe4O9 nanoparticles. Chem. Lett. 33(5), 502–503 (2004). https://doi.org/10.1246/cl.2004.502
J.T. Han, Y.H. Huang, R.J. Jia, G.C. Shan, R.Q. Guo, W. Huang, Synthesis and magnetic property of submicron Bi2Fe4O9. J. Cryst. Growth 294(2), 469–473 (2006). https://doi.org/10.1016/j.jcrysgro.2006.07.006
J. Luo, L. Wang, Z. Sun, P. Liu, Y. Lai, L. Zhu, H. Guo, Novel bismuth ferrite nanopowder prepared by polyethylene glycol-assisted two-step solid-state reaction: synthesis and magneto-optical properties. Ceram. Int. 47(3), 3514–3519 (2021). https://doi.org/10.1016/j.ceramint.2020.09.194
X. Yan, R. Pu, R. Xie, B. Zhang, Y. Shi, W. Liu, G. Ma, N. Yang, Design and fabrication of Bi2O3/BiFeO3 heterojunction film with improved photoelectrochemical performance. Appl. Surf. Sci. 552, 149442 (2021). https://doi.org/10.1016/j.apsusc.2021.149442
M. Fu, Z. Hongjian, Optical, magnetic, ferroelectric properties and photocatalytic activity of Bi2Fe4O9 nanoparticles through a hydrothermal assisted sol-gel method. Russ. J. Phys. Chem. A 93, 2079–2086 (2019). https://doi.org/10.1134/S0036024419100169
Funding
Jian Li reports financial support was provided by the National Natural Science Foundation of China (22168043). Long Yan reports financial support was provided by Science and Technology Plan Project of Shaanxi Province of China (2016KTZDGY08-04–01). Long Yan reports financial support was provided by Science and Technology Plan Project of Shaanxi Province of China (2020TD-031). Yufei Wang reports financial support was provided by Science and Technology Plan Project of Shaanxi Province of China (2021GY-165). Jian Li reports financial support was provided by Science and Technology Plan Project of Shaanxi Province of China (20JC039). Yufei Wang reports financial support was provided by the Nature Scientific Research Foundation of Shaanxi Provincial Education Office of China (21JP148). Long Yan reports financial support was provided by Joint Funds of Dalian National Laboratory For Clean Energy, CAS and Yulin University (LHJJ10).
Author information
Authors and Affiliations
Contributions
CX contributed to investigation, data curation, and writing of the original draft. YW contributed to the conception of the study. QW performed the data analyses and wrote the manuscript. JL performed the data analyses and wrote the manuscript. LY contributed significantly to manuscript preparation. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, C., Wang, Y., Wang, Q. et al. Phase transformation and heterojunction nanostructures of bismuth iron oxide. J Mater Sci: Mater Electron 34, 2236 (2023). https://doi.org/10.1007/s10854-023-11576-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11576-3