Abstract
The main goal of this study is to comprehend the effect of the composition change on crystallization characteristics, thermal, physical, and magnetic properties of glasses with the general formula (35 − x) Li2O − (5 + x) NiO − 60 P2O5 (x = 5, 10, and 15 mol%). The XRD results indicated that Li4P4O12, Li6P6O18, Ni2P2O7, Ni2P4O12, and Li3P crystalline phases were formed in the glass ceramics. The NiO/Li2O replacement led to an increase in the coherence of the glass structure. Density values for the glass and glass–ceramic samples ranged from 2.38 to 2.49 g/cm3 and from 2.41 to 2.53 g/cm3, respectively. Coefficients of thermal expansion CTE of the glasses were varied from 157 × 10−7 to 96 × 10−7 °C over the temperature range of 25–250 °C. However, CTE of the glass–ceramic was ranged from 183 × 10−7 to 129 × 10−7 °C over the temperature range of 25–500 °C. VSM analysis for the glasses revealed that the NiO/Li2O replacement led to a decrease in the magnetic parameters of the materials. Contrariwise from VSM hysteresis loops of glass–ceramic, it was found that the magnetic parameters increased with the addition NiO instead of Li2O. The results indicated a great potential of the prepared glasses and glass ceramics as promising soft ferromagnetic materials that can be used in different magnetic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, the research for obtaining new materials with unique physical and chemical properties has increased to meet the numerous and successive requirements of modern life. These materials derive their properties from their internal structure, diversity of atomic arrangements, and crystallized phases formed such as glass, ceramics, and glass–ceramic [1,2,3]. Glass and glass–ceramic materials based on phosphate possess many distinct properties for both fundamental and practical application. Phosphate-based glass and glass–ceramic materials exhibit low melting temperature, high thermal expansion coefficient, high ultra-violet transmission, dielectricity, and biocompatibility. Therefore, it has many applications in fields such as construction, electronics, biomedical, material science, and engineering [4,5,6]. Nowadays, interest in phosphate glass and glass–ceramic materials containing transition elements has increased. This is because its specific and distinct characteristics make them extremely appealing systems for an extensive variety of applications [7]. The transition metal oxides have a drastic effect on the structure and properties of the glasses, as well as play a pivotal role in the properties of the glass–ceramic materials containing an amount of it [7]. In previous studies, the effect of cobalt oxide as a transitional metal oxide and its effect on the structure and properties of formed glasses were studied [8, 9]. The properties of the cobalt-containing glasses are depended on their locations in the glass network, tetrahedral, and octahedral sites [10]. The effect of CoO on the composition, properties, and crystallization characteristics of glass based on lithium zinc metaphosphate was studied [7]. The effect of adding nickel at the expense of lithium in glass based on the nickel–lithium phosphate system on the structure, chemical, and physical properties was studied [11].
Recently, magnetic materials have attracted wide attention from researchers interested in materials science and its applications. This is due to its distinct properties that can be used in many applications such as energy storage, biomedicine, and optics [12, 13]. Among the many magnetic materials, magnetic glass–ceramic is a unique and distinct type due to its ease of preparation and its multiple and distinct properties [14, 15]. There are many systems that resulted from magnetic glass–ceramic materials, most of which are produced by melt-quenching glasses based on of iron (II) or iron (III) [16]. In order to prepare ferromagnetic glass–ceramic, there are only three transition metals (iron, nickel, and cobalt) that are ferromagnetic while other elements are either diamagnetic or paramagnetic [17]. The introduction of nickel oxide in glass compositions has attracted considerable attention for its possible applications in different fields [11, 18,19,20]. The magnetization of vanadium-phosphate glasses doped with nickel oxide has been studied [21]. The addition of nickel increased the magnetization of the glasses [21]. Influence of NiO on physical and magnetic properties of TiO2-doped magnesium ultra-Phosphate glass–ceramics was studied [22]. Significant increase in the magnetization is evidenced with the increase of NiO content in the crystalline materials [22]. The presence of large amounts of nickel in phosphate glass and its effect on crystallization and thermo-magnetic properties in literature is very limited. Therefore, the main goal of our study was investigated the effect of the NiO content on physical, thermal, and magnetic properties of glasses and glass–ceramic materials based on lithium nickel phosphate system with the general formula (35 − x) Li2O − (5 + x) NiO − 60 P2O5 (x = 5, 10, and 15 mol%) as a promising soft ferromagnetic materials.
2 Materials and methods
2.1 Glass preparation and crystallization
The glasses were synthesized by melt-quenching technique based on (35 − x) Li2O − (5 + x) NiO − 60 P2O5 (x = 5, 10, and 15 mol%) as shown in Table 1. The glass batches were prepared from chemical reagents (analytical grade) of Li2CO3 (≥ 99.0%), NH4H2PO4 (≥ 99.0%), and NiO (≥ 99.0%). All the designed batches were weighed and mixed well in a roll ball mill (TENCAN QM-5) for 2 h. The mixed powders were melted in alumina crucibles heated to 1050–1200 °C temperature range for one hour in an electrical furnace (Carbolite HTC 1550, United Kingdom). The vitreous melt was casted onto a stainless steel plate and then annealed at temperatures near Tg with a 10 °C/min heating rate for 5 h under air atmosphere conditions to remove residual thermal stresses. The synthesized glasses were named GCNi5, GCNi10, GCNi15, and GCNi20 as listed in Table 1. To convert glass into glass–ceramic without deformation, the crystallization process was carried out using data obtained from differential thermal analysis (DTA). In the crystallization process, the glass underwent two stages of heat treatment regime. Each glass sample was placed at endothermic and exothermic temperatures for five hours with a heating rate of 10 °C/min. After completing the two-stage heat treatment crystallization process, the oven was computerized closed to cool the crystalline samples to room temperature to start the characterization of the glass–ceramic samples.
2.2 Samples characterization
The appropriate temperatures for the crystallization process were accurately identified through the differential thermal analysis results (DTA) (SDTQ600—TA Instruments, USA). Through the DTA analysis, the glass transition and crystallization temperatures were identified with a heating rate of 10 °C/min from 25 to 1000 °C under an argon atmosphere of 60 mL/min. The phase composition of the quenched glasses and heat-treated glass ceramics were detected using X-ray diffraction (XRD) (TTR III, Rigaku Corporation, Japan) analysis in the angle range of 10° < 2θ < 90° at a scanning speed of 30°/min (working conditions: 30 kV, 30 mA). The morphology and phase distribution of the selected crystalline specimens were detected by using a scanning electron microscope (SEM; Quanta FEG 250, Netherlands). Before the analysis, the crystallized samples were chemically etched with a 1% HNO3 + 1% HF for 25 s to remove the residual glassy layer between the crystalline phases, and then the specimens were coated with a thin gold layer to obtain good conductivity surface.
The bulk density of the glass and glass–ceramic samples was measured using Archimedes’ method [23, 24]. To carry out the measurement, six samples were selected either from glass or from glass–ceramic materials. Xylene was used as the immersion solution (ρ = 0. 861 g/cm3). The thoroughness of the measurements is ± 0.01 g/cm3. The density was calculated using Eq. (1).
where Wa is the weight of the specimen in air, Wb is the weight of the specimen in xylene, and ρx is the density of xylene.
The coefficient of thermal expansion (CTE) of the prepared glass and glass–ceramic specimens was determined using Netzsch DIL 402C horizontal dilatometer with a heating rate of 10 °C/min under air atmospheres. A rectangular specimen with dimensions of (5.0 × 5.0 × 20 mm) was formed by cutting and polishing to obtain parallel faces. The magnetic parameters for the glass and glass–ceramic samples were determined using vibrating sample magnetometer equipment (VSM, Lake Shore, 7410, USA) at room temperature with the applied field of ± 20 kOe. Through the magnetization curves, saturation magnetization (Ms), remanence magnetization (Mr), and coercivity (Hc) were determined.
3 Results and discussion
3.1 Crystallization characteristics
3.1.1 Thermal behavior of the glasses
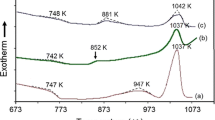
Crystallization behavior of the prepared glasses including thermal performance, phases created, and microstructure of the controlled heated samples were studied. It is well known that the preparation of glass–ceramic materials requires the formation of the crystalline phases from the amorphous phase at certain temperatures. Subsequently, it is very essential to explore these temperatures macroscopically by DTA or DSC (differential scanning calorimetry) technique. Figure 1a shows the DTA examination results of the prepared glasses at the heating rate of 10 °C/min. From the DTA curves, the transition temperature Tg, crystallization temperature Tc, and thermal stability ΔT (where ΔT = Tc − Tg) of the glasses are estimated as shown in Fig. 1b and recorded in Table 1. All curves showed perfect shapes and consisted of one endothermic peak and one exothermic peak, which corresponded to the glass transition and crystallization temperatures, respectively. The endothermic peaks were in the range of 281–400 °C, while the exothermic effects were in the 661–683 °C temperature range and ΔT of the glasses was recorded the range of 272–380 °C. The DTA results of the base glass (GCNi5), Fig. 1a, presented that an endothermic peak effect happened at 281 °C and one pronounced exothermic peak occurred at 661 °C. As can be seen from Fig. 1b and Table 1, the glass transition (Tg) and the crystallization temperatures (Tc) of glasses increased with the increase of NiO/Li2O replacement ratios. The increase in Tg and Tc temperatures may be attributed to the increase of NiO content in the glass samples. Also, there was a decrease in non-bridging oxygen due to reduction of the amount of network-modifying ions, which would restrain the destruction of [Si–O] networks [25]. On the other hand, nickel changes valency in vitreous structures from NiO6 octahedral to NiO4 tetrahedral leading to an increase in the strength of the glass network and hence an increase in the values of transition and crystallization temperatures [11, 20, 26]. One of the important parameters assigned to glass–forming ability is the glass thermal stability (ΔT). The thermal stability of glass greatly controls the glass-forming ability and quality of the nucleation and crystallization procedures [14, 27]. The change in the composition of the glasses and its effect on the change in the thermal stability of the prepared glasses against devitrification were studied. The lower value of the ΔT was represented lower thermal stability and higher crystallization propensity [28]. The thermal stability values of the prepared glasses GCNi5, GCNi10, GCNi15, and GCNi20 were 380, 370, 319, and 272 °C, respectively, as presented in Fig. 1b and Table 1. It was clear from the thermal stability results that the stability of the base glass decreased and nucleation and crystallization processes were enhanced by the addition of NiO at the expense of Li2O.
3.1.2 Crystalline phases formed and microstructure
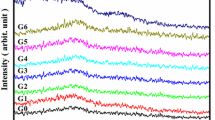
Generally, transforming glass into crystalline glass–ceramic was carried out by subjecting the glass to two stages of controlled heat treatment including nucleation and crystals growth. The crystal growth process gains a specific importance because it controls the type and morphology of the formed crystalline phases [14, 29]. The crystallization phases formed and the microstructure of the controlled crystallized glasses were carefully studied. The XRD patterns of the crystallized glasses with different content of NiO and Li2O are shown in Fig. 2. It is noticeable from the results of XRD that the formed crystalline phases were changed dramatically with the change of NiO/Li2O replacement ratio. From Fig. 2, it was indicated that the main crystalline phases formed in the base glass GCNi5 were Li4P4O12 (PDF# 39-0122), Li6P6O18 (PDF# 89-0858), and Ni2P2O7 (PDF# 83-1355) as minor phases. Since the discovery of glass–ceramic in the early 1960s, researchers have paid great attention to study the crystallization mechanism of different glass families, such as silicate, telluride, borate, and phosphate. In the phosphate glasses, glassy material tends to crystallize spontaneously. Once crystallization occurred, pyrophosphate glass would be crystallized in both orthophosphate and metaphosphate crystals [30, 31]. The effect of NiO/Li2O ratios on the phases formed in the crystalline materials was attained by the XRD analysis (Fig. 2). Controlled heat treatment of the glass GCNi10 (with 10 mol% NiO) led to the formation of nickel metaphosphate phase Ni2P4O12 (PDF# 076–1557) at the expense of Li6P6O18 phase as proved by the XRD (Fig. 2, patterns I and II). By increasing the addition of NiO instead of Li2O up to 15 mol% (sample GCNi15), the XRD analysis (Fig. 2, pattern III) clearly showed that Ni2P4O12 (PDF# 076-1557) and Li3P (PDF# 74-1160) phases were formed (Fig. 2 patterns III). At high NiO replacement, 20 mol% (sample GCNi20), the XRD analysis (Fig. 2, pattern IV) indicated that nickel metaphosphate Ni2P4O12 was developed as a main phase at the expense of Li3P phase. Metaphosphates-based materials, such as nickel metaphosphate (Ni2P4O12), are very important, where they can be used as stable electrocatalyst [32]. The crystallization of nickel metaphosphate crystals may be due to the combination of equivalent amount of nickel with phosphorus pentoxide to form the nickel metaphosphate phase (Eq. 2).
SEM micrograph of the controlled crystallized glasses (Fig. 3) showed the influence of adding NiO at the expense of Li2O on the microstructure of the studied crystalline materials. The morphology of crystallized specimens presented that the microstructure of samples became more compact and the grain size decreased with the increase of NiO/Li2O. This may be attributed to an increase in the viscosity of glass which led to a decrease in the crystal size of the crystallized phases [33, 34]. Consequently, compared with the base sample, GCNi5, the microstructure of crystallized phases in GCNi20 crystallized specimen becomes more compact and tiny. SEM micrograph of GCNi5 base specimen (Fig. 3) showed volume crystallization of prismatic-like growth crystals, while, at 20 mol% NiO, fine fibrous crystals were formed as shown in Fig. 3b. The decrease in crystal size with the addition of NiO is completely consistent with the results obtained by researchers [20, 35]. The addition of NiO with diverse quantities led to oriented crystallization of crystals in one direction as needle-like growths [20, 35]. It was also renowned that the crystallization procedure was precise linearly with increase in the amount of nickel in the glasses [20, 35].
3.2 Materials characterization
3.2.1 Density
In many cases, the interpretation of the change in the strength or weakness of synthesized glasses structures depends on the results of the density (ρ) of the glasses. Density values of the glass materials depend mainly on the percentage of oxides in the glass mixture in addition to the molecular weight of those oxides. In this part, the effect of replacing lithium oxide with nickel oxide on the change in density of prepared glass samples will be explained. The density results of the glasses and glass–ceramic specimen are shown in Fig. 4 and summarized in Table 2. The results proved that the addition of nickel at the expense of lithium oxide increased the density of the prepared glasses. As NiO was systematically substituted, the density was increased from 2.38 to 2.50 g/cm3 with an increasing NiO up to 20 mol%. The increase in density with the increase of nickel in the glass samples can be attributed to the increase in the compactness of the internal glass structure [11, 36]. The decrease in the percentage of alkaline oxides in the glass, such as sodium and lithium, works increase the strength of the glass network, and so, an increase of the density of the prepared glasses [37, 38]. On the other hand, the increase in the overall weight of the glass, as the atomic weight of nickel oxide NiO (74.69 g/mol) is higher than the atomic weight of lithium oxide (29.88 g/mol) ,led to an increase in the density values of the prepared glasses. The bulk density of the controlled crystalline glasses is shown in Fig. 4 and Table 2. The density data showed that the density values of the glass–ceramic specimens were in the range of 2.41–3.53 g/cm3. It is clear from the results that the crystallization led to an increase in the density values of the glass–ceramic samples than that of the corresponding glass specimens. The density of glass–ceramic is affected by a number of factors, and the most important ones are as follows: the main components, heat treatment schedule, crystals formed, and microstructure [39]. It is also found that the glass–ceramic densities were increased with the increase of nickel oxide. This may be due to the formation of fine microstructure and high atomic mass crystalline phases with increasing the NiO/Li2O replacement. Higher bulk density means condensed microstructure of the glass–ceramics and less pores in crystalline materials [39, 40].
3.2.2 Coefficients of thermal expansion CTE
The determination of the thermal expansion coefficient of the glass is one of the important properties that determine the potential application of the prepared glass. There are many factors that greatly affect the thermal properties of glass, especially thermal expansion, such as composition, network structure, bond toughness, nature and contribution of the different cations, and annealing behavior of the prepared glasses [41, 42]. The thermal expansion behavior of the glass samples prepared in this study based on nickel lithium phosphates glass system was studied as shown in Fig. 5. This study was carried out in the thermal range from room temperature up to 500 °C with a thermal rate 10 °C/min. All the prepared glass samples GCNi5, GCNi10, GCNi15, and GCNi20 show the ideal shape of the thermal expansion curve of the amorphous materials' positive thermal expansion. The base crystalline sample GCNi5 shows the highest α-value in addition to the lowest transition and softening temperatures. The results showed that adding NiO at the expense of Li2O resulted in a decrease in the CTE values, and an increase in Tg, as well as Ts temperatures. This can be attributed to the addition of nickel led to an increase in the tightness of the glass lattice. Generally, nickel is present in the glass lattice in two places octahedral NiO6 or tetrahedral NiO4 [11, 18]. With an increase in the proportion of nickel in the composition, nickel prefers to switch from octahedral to tetrahedral sites which led to increasing the tightness and joining degree of the glass network [11, 18].
In many cases, the thermal expansion coefficient of glass–ceramic materials differs from those of the parent glasses. Sometimes, the crystallization process leads to an increase in the coefficient of thermal expansion, and at other times, it may lead to a decrease in the coefficient of thermal expansion until it reaches negative values [43]. The values of the thermal expansion coefficient of glass–ceramic are mainly reflected in the crystalline phases formed and the residual glassy matrix [14, 38, 44]. It is known that increasing the residual glass content in glass–ceramic increases its coefficient of thermal expansion [14]. In our study, the prepared glasses were exposed to a controlled heat treatment process to obtain holocrystalline materials without deformation. In Fig. 6 and Table 2, the data obtained from the thermal expansion coefficient of the studied crystalline samples are presented. The synthesized glass–ceramics showed α-values varying from 129 to 183 × 10–7 in the temperature range between 25 and 500 °C with 10 °C intervals. The base glass–ceramic specimen demonstrated the highest thermal expansion value of 183 × 10–7. The CTE results also showed that the addition of nickel instead of lithium reduced the thermal expansion coefficient of the crystalline materials (Fig. 6; Table 2). This could be attributed to the crystallization of a relatively low thermally expanding nickel metaphosphate Ni2P4O12 phase instead of relatively high thermally expanding Li4P4O12 and Li6P6O18 phases.
3.2.3 Magnetic characterization
The VSM magnetization behavior of the prepared lithium nickel phosphate glass and glass–ceramic materials at a magnetic field of 20 kOe is shown in Figs. 7, 8 and Table 2. In this study, we focus on the impact of nickel amount on magnetic properties of synthesized glasses and glass–ceramic materials. All the glass and crystalline specimens exhibited a soft ferromagnetic behavior. A set of important magnetic parameters were determined from the VSM hysteresis loops results, for example, saturation magnetization (Ms), remanence magnetization (Mr), and coercivity (Hc). Figure 7 displays the difference in magnetization of glass samples with the function of NiO/Li2O content. The coercivity (Hc), saturation magnetization (Ms), and the remanence magnetization (Mr) values of the glass samples are 120, 106, 70; 53 G, 0.768, 0.593; 0.541, 0.489, and 4.04; 3.10, 2.13, and 1.97 emu/g for GCNi5, GCNi10, GCNi15, and GCNi20, respectively. It is clear from the magnetism results of the glass samples that magnetic parameters decrease with the increase of nickel content and particularly the specimen with 20 mol% NiO, a sample GCNi20. These results are consistent with the previous study of Ni-doped cobalt molybdenum phosphorus borate glasses [39]. The decrease of magnetism with increasing the nickel contents may be due to the increase of precise capacitance of prepared glasses. The values of the magnetic properties of the prepared glasses indicated that they can be used in promising applications such as cathodes for energy storage applications or super-capacitor cathodes [45]. Figure 8 clarifies VSM hysteresis loops of the prepared glass–ceramic specimen. The results showed that the base crystalline sample GCNi5 has the lowest (Ms), (Mr), and (Hc) values compared to all crystallized specimens. It is clear from the results that all the magnetic parameters that were determined were increased with the replacement of lithium oxide with nickel oxide Fig. 8 and Table 2. The magnetic properties of crystalline materials, especially glass ceramic, are often affected by a number of factors, including phases formed, microstructure, composition of the residual glassy matrix, and the applied magnetic field [46,47,48].
The magnetic results of the glass–ceramic samples show that the replacement of Li2O with NiO led to enhance saturation magnetization as compared with the base crystalline sample. This may be due to crystallization and increased concentration of nickel metaphosphate phase Ni2P4O12 instead of Li4P4O12 and Li6P6O18 phases as observed from the XRD patterns Fig. 2. The saturation magnetization (Ms) value of controlled heat-treated glasses was mainly depend on the crystalline phases formed [46,47,48,49,50]. On the other hand, there was a noticeable increase in both coercivity and remanence magnetization parameters with NiO/Li2O replacements (Fig. 8; Table 2). This may be attributed to the formation of fine-grained microstructure in the glass–ceramics with the addition of NiO instead of Li2O as shown in Fig. 3. There are many factors that strongly affect the coercivity and remanence magnetization of the materials such as microstructure, size, and shape of the phases formed and the applied magnetic field [51]. As the crystallite size decreased, the coercivity and remanence magnetization parameters are increased [52, 53].
4 Conclusions
A series of glass and glass–ceramics materials based on lithium nickel phosphate system with the general formula (35 − x) Li2O − (5 + x) NiO − 60 P2O5 (x = 5, 10, and 15 mol%) were prepared. The addition of NiO instead of Li2O significantly affected the physical, thermal, and magnetic properties of the synthesized glass and glass–ceramic materials. The increase in NiO/Li2O replacement led to an increase in the coherence of the glasses structure and an increase in the density from 2.38 to 2.49 g/cm3. It also led to an increase in the density of the glass ceramics from 2.41 to 2.53 g/cm3. Coefficients of thermal expansion (CTE) of the glasses are varied from 157 × 10−7 to 96 × 10−7°C over the temperature range of 25 − 250 °C. However, CTE of the glass–ceramic is ranged from 183 × 10−7 to 129 × 10−7 °C over the temperature range of 25 − 500 °C. The VSM results showed that the process of replacing lithium with nickel led to the weakening of the magnetic properties of the glasses. On the contrary, the magnetic properties of the synthesized glass–ceramics showed a significant increase with the increase in the content of nickel in the samples. Results indicated a great potential of the prepared glass and glass–ceramic as soft magnetic materials.
References
R. Alves, A. Silva, N. Dantas, A. Gouveia-Neto, J. Lumin. 230, 117738 (2021). https://doi.org/10.1016/j.jlumin.2020.117738
M. Sayyed, M. Abdo, H.E. Ali, M. Sadeq, Ceram. Int. 48, 30817–30825 (2022). https://doi.org/10.1016/j.ceramint.2022.07.035
H. Zhang, J. Liu, F. Shi, H. Zhang, X. Yuan, Y. Li, T. Li, X. Zhao, M. Wang, Ceram. Int. (2022). https://doi.org/10.1016/j.ceramint.2022.08.331
B. Demir, D. Derince, T. Dayioglu, L. Koroglu, E. Karacaoglu, V. Uz, E. Ayas, Ceram. Int. 47, 34657–34666 (2021). https://doi.org/10.1016/j.ceramint.2021.09.004
A. Rout, S. Agrawal, Ceram. Int. 47, 11702–11713 (2021)
H. Chen, Q. Sun, J. Zhang, J. Sheng, Ceram. Int. 48, 20400–20408 (2022). https://doi.org/10.1016/j.ceramint.2022.03.325
M. Jerroudi, L. Bih, S. Yousfi, B. Manoun, P. Lazor, Physica B 610, 412949 (2021)
A. Abdelghany, G. El-Damrawi, A. Oraby, M. Madshal, Physica B 573, 22–27 (2019)
A. Abdelghany, G. El-Damrawi, A. Oraby, M. Madshal, J. Non-Cryst. Solids 499, 153–158 (2018)
M. Kharroubi, H. Assad, S. Balme, L. Gacem, C. Maghni, Ionics 22, 2355–2361 (2016)
H. Abo-Mosallam, E.A. Mahdy, J. Cent. South Univ. 29, 1437–1446 (2022). https://doi.org/10.1007/s11771-022-5033-6
A. Kozlovskiy, D. Shlimas, M. Zdorovets, J. Mater. Sci.: Mater. Electron. 32, 12111–12120 (2021)
T.G. Avancini, M.T. Souza, A.P.N. de Oliveira, S. Arcaro, A.K. Alves, Ceram. Int. 45, 4360–4367 (2019). https://doi.org/10.1016/j.ceramint.2018.11.111
H. Abo-Mosallam, E.A. Mahdy, J. Non-Cryst. Solids 583, 121467 (2022)
X. Du, Y. Pu, X. Li, X. Peng, Z. Sun, J. Zhang, J. Ji, R. Li, Q. Zhang, M. Chen, Ceram. Int. 47, 8987–8995 (2021). https://doi.org/10.1016/j.ceramint.2020.12.021
O. Sedighi, A. Alaghmandfard, M. Montazerian, F. Baino, J. Am. Ceram. Soc. 105, 1723–1747 (2022)
S. Danewalia, K. Singh, Mater. Today Bio 10, 100100 (2021)
B. Johnson, N. Chand, B. Sudhakar, G. Srinivasa Rao, J. Mater. Sci.: Mater. Electron. 27, 8833–8847 (2016)
A.V. Sekhar, L. Pavić, A. Moguš-Milanković, V.R. Kumar, A.S.S. Reddy, G.N. Raju, N. Veeraiah, J. Alloys Compds. 824, 153907 (2020)
H. Abo-Mosallam, S. Ibrahim, E.A. Mahdy, J. Non-Cryst. Solids 580, 121385 (2022)
G. Khattak, V. Keith, P. Martin, E. Khawaja, M. Khan, J. Magn. Magn. Mater. 94, 278–286 (1991)
S. Abd-Rahman, M. Sahar, S.K. Ghoshal, Adv. Mater. Res. 1107, 32–37 (2015)
M.R. Ahmed, M. Shareefuddin, SN Appl. Sci. 1, 1–10 (2019)
K.C. Sekhar, B. Srinivas, N. Narsimlu, M.N. Chary, M. Shareefuddin, Mater. Res. Express 4, 105203 (2017)
W. Shang, Z. Peng, F. Xu, H. Tang, M. Rao, G. Li, T. Jiang, Ceram. Int. 47, 29400–29409 (2021). https://doi.org/10.1016/j.ceramint.2021.07.108
S. Ibrahim, H. Abo-Mosallam, E.A. Mahdy, G.M. Turky, J. Mater. Sci.: Mater. Electron. 33, 10596–10610 (2022)
K. Joseph, T. Ravindran, R. Sudha, R. Asuvathraman, J. Nucl. Mater. 517, 106–112 (2019)
J. Liu, K. Yang, J. Zhai, B. Shen, H. Wang, F. Li, Mater. Chem. Phys. 206, 29–34 (2018). https://doi.org/10.1016/j.matchemphys.2017.12.003
H.A. Abo-Mosallam, M. Farag, J. Aust. Ceram. Soc. 56, 353–361 (2020)
Y. Chen, S. Liu, Y. Zhou, P. Shang, Z. Shan, J. Zhang, J. Non-Cryst. Solids 584, 121505 (2022). https://doi.org/10.1016/j.jnoncrysol.2022.121505
A. Berezicka, M. Szumera, J. Sułowska, P. Jeleń, Z. Olejniczak, J. Stępień, M. Zając, S. Pollastri, L. Olivi, Ceram. Int. 48, 4238–4254 (2022). https://doi.org/10.1016/j.ceramint.2021.10.216
J. Wang, J. Hu, S. Niu, S. Li, Y. Du, P. Xu, Small 18, 2105972 (2022)
R. Jia, L. Deng, F. Yun, H. Li, X. Zhang, X. Jia, Mater. Chem. Phys. 233, 155–162 (2019). https://doi.org/10.1016/j.matchemphys.2019.05.065
Y. Hou, G.-H. Zhang, K.-C. Chou, D. Fan, J. Non-Cryst. Solids 538, 120023 (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120023
M. Majerová, A. Prnová, A. Plško, B. Hruška, J. Valúchová, J. Kraxner, E. Bruneel, K. De Buysser, D. Galusek, J. Therm. Anal. Calorim. 142, 2111–2121 (2020). https://doi.org/10.1007/s10973-020-10154-7
H.A. Abo-Mosallam, S. Ibrahim
H. Abo-Mosallam, E.A. Mahdy, J. Non-Cryst. Solids 546, 120279 (2020)
M. Wei, F. He, X. Cao, B. Zhang, C. Zheng, J. Xie, Ceram. Int. 48, 27718 (2022)
X. Ma, Q. Li, L. Xie, C. Chang, H. Li, J. Non-Cryst. Solids 587, 121573 (2022). https://doi.org/10.1016/j.jnoncrysol.2022.121573
Z. Li, G. Ma, D. Zheng, X. Zhang, R.A. Muvunyi, Ceram. Int. 48, 21245 (2022)
H. Abo-Mosallam, J. Non-Cryst. Solids 571, 121084 (2021)
M. Salinigopal, N. Gopakumar, P. Anjana, O. Pandey, J. Non-Cryst. Solids 535, 119956 (2020)
W. Holand, G.H. Beall, Glass-Ceramic Technology (Wiley, Hoboken, 2019)
Y. Yang, H. Ren, H. Peng, T. Xie, Y. Zhang, F. He, H. Lin, Ceram. Int. 48, 27913–27921 (2022). https://doi.org/10.1016/j.ceramint.2022.06.095
A. Montreeuppathum, P. Kidkhunthod, S. Rujirawat, R. Yimnirun, S. Pinitsoontorn, S. Maensiri, Radiat. Phys. Chem. 170, 108677 (2020). https://doi.org/10.1016/j.radphyschem.2019.108677
S.R. Mortazavi, F. Karimzadeh, R. Emadi, H. Ahmadvand, J. Non-Cryst. Solids 559, 120704 (2021). https://doi.org/10.1016/j.jnoncrysol.2021.120704
H. Feng, J. Huang, X. Wang, J. Li, X. Yin, Z. Xu, K. Xiong, Ceram. Int. 48, 9090–9098 (2022)
M.M. Shakdofa, E.A. Mahdy, H. Abo-Mosallam, Ceram. Int. 47, 25467–25474 (2021)
S. Salman, S. Salama, H. Abo-Mosallam, Ceram. Int. 42, 8650–8656 (2016)
M. Abbasi, B. Hashemi, H. Shokrollahi, J. Magn. Magn. Mater. 356, 5–11 (2014)
B.D. Cullity, C.D. Graham, Introduction to Magnetic Materials (Wiley, Hoboken, 2011)
O. Bretcanu, S. Spriano, E. Verné, M. Cöisson, P. Tiberto, P. Allia, Acta Biomater. 1, 421–429 (2005)
S. Salman, S. Salama, H. Abo-Mosallam, Ceram. Int. 41, 1521–1529 (2015)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abo-Mosallam, H.A., Farag, M.M. The impact of NiO on crystallization and thermo-magnetic properties of Li2O–NiO–P2O5 glasses as new magnetic materials. J Mater Sci: Mater Electron 34, 602 (2023). https://doi.org/10.1007/s10854-022-09749-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09749-7