Abstract
Polycrystalline samples of (Bi1-xInx)2Se2.7Te0.3 (x = 0.00, 0.02, and 0.04) were prepared by the solid-state reaction technique. X-ray diffraction pattern confirms that the polycrystalline samples have a hexagonal structure with spacegroup R \(\stackrel{-}{3}\) m. The surface morphologic study reveals the existence of porous behavior in the studied samples due to the volatilization of Selenium. Energy dispersive X-ray analysis validates the expected and observed elemental composition of the samples. Electrical resistivity has shown metallic behavior. Hall effect and Seebeck coefficient measurements indicate the p-type and n-type conduction for the pristine sample Bi2Se3 and the (Bi1-xInx)2Se2.7Te0.3 samples, respectively. The thermal conductivity and electrical resistivity were found to reduce by 7.5 and 9 times, respectively, for (Bi0.96In0.04)2Se2.7Te0.3 compared to the pristine sample Bi2Se3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the best solutions for renewable energy is thermoelectric (TE) materials, which convert waste heat into electricity and vice versa and provide better management for clean and green energy production [1]. The efficiency of thermoelectric material is determined by a dimensionless quantity called the thermoelectric figure of merit (ZT) and is calculated using the formula \(ZT=\frac{{S}^{2}T}{\kappa \rho }\), where S is the Seebeck coefficient, κ is the total thermal conductivity, ρ is the electrical resistivity, and T is the absolute temperature [2, 3]. The chalcogenide materials like Bismuth Selenide and Bismuth Telluride are the reliable low-temperature TE materials that are used for Peltier cooling devices. The alloys based on Bismuth Selenide and Bismuth Telluride have been studied extensively as representative materials for thermoelectric applications near room temperature. The most challenging task in search of high-performance TE materials is to simultaneously control the electrical conductivity and thermal conductivity [4].
Adam et al. [5] have obtained the power factor of 131 × 102 μW/mK2 for 2D material Bi2Se3-xTex. Ali et al. [6] have reported Bi2Te2.7Se0.3 tetradymite by the physical vapor deposition method. Kadel et al. [7] have reported Bi2Se3 thin film with ZT of 0.096 at 523 K, synthesized by nanoscale route method. Quentin et al. [8] have reported the thermoelectric performance of Cu-doped n-type Bi2Te3 with ZT of 0.1 at 300 K. Margarita et al. [9] have reported vapor–solid synthesis of Bi2Se3 with the fabrication of graphene layer. Ramezani et al. [10] have introduced the nanoparticle synthesis of Bi2Se3 as a novel precursor. Nano flakes of Bi2Se3 have been reported with a ZT value of 0.02 at 300 K by Yuho et al. [11]. Single crystal Bi2Se3 was reported by Anuj Krishna et al. [12] with ZT of 0.6 at 400 K. Kulsi et al. [13] have reported Ni-doped Bi2Se3 with ZT value of 0.22 at 300 K. Enhanced thermoelectric properties of phase-separating bismuth selenium telluride thin films via a two-step method was reported by Masayuki et al. [14]. Jana et al. [15] reported the thermoelectric properties of Bi2Se3 thin film with the Seebeck coefficient of 150 μV/K by the vapor–solid technique. Thermoelectric properties of Bi2Te3 doped with different copper amounts have been reported by Lognone et al. [16]. From the literature mentioned above, the effects of co-doping are much less studied in the chalcogenide thermoelectric materials in the low and near room temperature range (10–350 K).
Since Bi2Se3 is bonded with Van der Waals force between layers, the extra layer should be introduced to stack Bi2Se3 superlattice. The introduction of Indium will produce an extra electron to the Bi2Se3 lattice, in which an increase in the carrier concentration is expected [17]. The effect of Indium substitution may lead to the localized electronic states in crystal, which would vary the electronic properties of the alloys. In the (Bi1-xInx)2Se2.7Te0.3 system, the Bismuth ions are larger than Indium ions, and the Tellurium ions change the c axis spacing by creating SeTe vacancies. Hence, the co-doping in Bi2Se3 will significantly modify the structural parameters and in turn, the thermoelectric properties [18]. Tellurium can achieve a high level of substitution on the Selenium site due to their similar chemical properties [19]. The doping of Tellurium in the site of Selenium finds an active approach to avoid the intrinsic conduction. Also, it seems to balance the carrier concentration and resultant enhancement of thermoelectric properties in Bi2Se3. As an optimization, lattice thermal conductivity can be reduced by intensified scattering of alloys for electrons and phonons [20]. The co-substitution of Bi by In favors the construction on TeSe antisite defect of Bi2Se3. The challenging task is to the decrease in thermal conductivity without deteriorating the electrical conductivity, which motivates us to study co-doping effects in chalcogenides. The present investigation is focused on the thermoelectric properties of the (Bi1-xInx)2Se2.7Te0.3 system in the temperature range 10–350 K. Remarkably, the simultaneous decrease of electrical resistivity and thermal conductivity is observed in the co-doped chalcogenides, and in turn, an enhancement of the ZT value is achieved.
2 Experimental procedure
2.1 Sample preparation
The samples of Bi2Se3 and (Bi1-xInx)2Se2.7Te0.3 were synthesized by solid-state reaction technique in which the precursors Bismuth (99.99%), Indium (99.9%), Selenium (99.995%), and Tellurium (99.99%) were added in a stoichiometric ratio and mixed well in an agate mortar for 2 h with an intense grinding. The mixed powder was subjected to pelletization under the compression pressure of 5 tons. The pellets of dimension 10 × 2 × 5 mm3 were obtained. The pellets were sealed in a quartz tube under a vacuum of 10–6 torr and sintered at 480 °C for 30 h. The grinding is repeated for the sintered pellets to improve the homogeneity in the compounds. The pelletization is repeated and sintered at 250 °C for 15 h.
2.2 Experimental techniques
The X-ray diffraction (XRD) study was carried out by powder X-ray diffractometer (Rigaku Miniflex with Cu Kα) in the angle 2θ range 22°–80° at the rate of 2°/min to confirm the purity, crystallinity, dominated phase, and formation of compounds. The surface morphological features of samples were inspected by field emission scanning electron microscope (Carl Zeiss Sigma) at the particle range of 1 μm with the magnification of 35 kX. The chemical composition of grown samples was examined by energy dispersive X-ray analysis (EDAX) using EVO MA18 with Oxford EDS (X-act).
The carrier concentration, mobility, and Hall coefficient at room temperature were measured by the Van der Pauw technique. The low and near room temperature electrical resistivity was measured in the temperature range 10 – 350 K using the four-point probe method in closed cycle refrigeration. The dc heat pulse technique was used to measure the Seebeck coefficient (S) and thermal conductivity (κ) simultaneously [21].
3 Results and discussion
3.1 X-ray diffraction (XRD) study
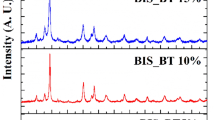
Figure 1 shows the XRD patterns of the samples Bi2Se3 and (Bi1-xInx)2Se2.7Te0.3. The highest intensity peak (015) has been observed for the samples Bi2Se3, (Bi0.98In0.02)2Se2.7Te0.3 and (Bi0.96In0.04)2Se2.7Te0.3, whereas the peak (216) is dominated in Bi2Se2.7Te0.3 sample. This could be due to the variation in the recrystallization of the sample during the sintering and anisotropic nature of the crystal [22]. Besides, there is a gradual shifting in the peak position to the lower 2θ angle side, except the sample doped with In (x = 0.04) (Fig. 2). The shift in XRD peak position is due to the insertion of the larger ionic radius atom tellurium onto the smaller ionic radius of Selenium site. The random variation in the sample doped with In (x = 0.04) is because of uneven variation of strain in the sample, which has affected the crystallite size, lattice parameter, and is listed in Table 1. The crystallite size has been calculated using the Williamson Hall formula [23].
where β is full width half maximum intensity, \(\uplambda\) is the wavelength of X-ray, ε is the strain, and D is the crystallite size. It is observed that there is a gradual increase in the crystallite size, in line with an increase in the lattice parameter values. The twinning effect is observed at 28° and 45° for all the samples, which corresponds to the sharing of the lattice point on the face of the existing crystal. The XRD peak patterns are fitted by the software “EXPO 2014” which is shown in Fig. 3 [24, 25]. The values of Rp (Profile factor), Rep (Expected Profile factor), Rwp (Weighted profile factor), and χ2 values have been tabulated in Table 1. The refined XRD patterns show that the crystal belongs to the hexagonal crystal system with R \(\stackrel{-}{3}\) m space group.
3.2 Field emission scanning electron microscopy (FESEM)
Figure 4a shows the surface morphology of the pristine Bi2Se3 sample. It is observed that there are growth and shrinkage of the grains in some areas without any sign of impurities. As the Tellurium is inserted into the matrix of Bi2Se3, the white patches can be observed in certain areas with some porous behavior, as shown in Fig. 4b and c. Besides, there is an increase in the porosity of the In-doped sample (x = 0.04) due to the volatilization of Selenium during sintering [Fig. 4d]. Partial evaporation of Selenium causes decrease in the concentration of \({V}_{Se}^{..}\). As a result, the electron concentration decreases. Hence there is a variation in the density of the material in all the compounds [26, 27]. The selenium vapor pressure increases during sintering which forms negative gradient of Se precipitates on the surface. The effect of doping of Te has resulted in the agglomeration of the particles [28, 29].
3.3 Energy dispersive X- ray analysis (EDAX)
The chemical composition of the samples was examined by the EDAX, and the images of the studied samples are shown in Fig. 5. The pristine Bi2Se3 sample indicates the presence of Bismuth and Selenium as expected, whereas the Bi2Se2.7Te0.3 sample shows the presence of Tellurium along with the Bismuth and Selenium. For the (Bi1-xInx)2Se2.7Te0.3 samples, the presence of Indium in these In-doped samples (Fig. 5c, d) is confirmed. The observed elemental composition of the studied samples matches well with the expected pre-crystal formation, as shown in Table 2.
3.4 Electrical resistivity
The temperature-dependent electrical resistivity ρ(T) of the (Bi1-xInx)2Se2.7Te0.3 system is shown in Fig. 6. It is seen that the pristine sample has the highest electrical resistivity among all studied samples, while the In-doped (x = 0.04) sample has the lowest electrical resistivity. It is noticed that there is a gradual decrease in electrical resistivity with the increase in the doping level. Initially, the pristine sample shows the usual semiconductor behavior, in which the resistivity decreases as the temperature increases till 233 K and metallic behavior is observed after 233 K. This could be due to the direct cation–cation interaction of homopolar bonds of Bi-Bi, which changes the bandgap as temperature changes [30]. The donor band of the quantum states are occupied by the fifth electron of donor atom. There is an overlapping of donor band with the conduction band which leads to the composite band. The few of the lower energy states of this band is occupied by electrons, whereas other band energy states are unfilled. The insertion of In into the site of Bi has caused the intrinsic point defect which is dependent on the polarity of the bond [31]. Due to the differences in the electronegativities, there is a suppression of bonding between Bi-Se by In-Se. Hence there is a probability of bonding between In-Se and In-Te rather than Bi-Te and Bi-Se. As a result, electrical resistivity increases, with increase in temperature. Along with this, there is a decrease in \({V}_{Te}^{..}\) and \({V}_{Se}^{..}\) vacancies with the change in the creation of energy lattice defect in mixed crystal Bi1-xInxSe3-xTex. Also, at greater temperature, electrons are captured from the conduction band. The doping of In in Bi2Se3 also initiates the volatilization of Se and Te atoms during sintering. Hence the samples Bi2Se2.7Te0.3, (Bi0.98In0.02)2Se2.7Te0.3, and (Bi0.96In0.04)2Se2.7Te0.3 show degenerate semiconducting behavior [32, 33]. The drastic change in electrical resistivity from pristine to the doped samples is presumably due to impurity band, which makes disorders in the potential energy of the donors [34]. Fermi level is situated in the middle of the band gap, although there is a huge intrinsic vacancy, Bi2Se3 has high hole concentration. Fermi level lies just above the valence band, which results in p-type transport behavior in Bi2Se3, where the total density of states is varied unconditionally. Deep defect states are basically filled in bulk band in Bi2Se3. This probable distortion of density of states occurred due to doping of indium in valence band region. As a result, there is a creation of deep defect states above the valence band. Also, the hybridization of In with Bi is also the reason for deep defect states [35]. The distorted density of states is initiated by the interaction of pristine with the dopant indium atom. This variation in defect states bring the modification in electrical resistivity. Along with the doping of indium, it is observed that tellurium doping leads to an increase in the density of ionized impurities, as the dopants concentration become large due to co-doping. Te has been introduced to increase carrier concentration and scattering of carriers through SeTe vacancies. The influence of carriers excited by the doping of tellurium results in the increase of electrical conductivity. SeTe atoms occupy BiIn vacancies resulting in the formation of antisite defects. These antisite defects produce excess of electrons. They will be excited from valence band to the conduction band easily by the doping of tellurium [36,37,38]. This phenomenon could be enhanced even at the time of sintering. As a result, the antisite defect is formed in the doped and co-doped samples [39]. This leads to the super-stoichiometric behavior of the Bi(In)–Bi(In), which replaces the SeTe sublattice. The lattice point defect in (Bi1-xInx)2Se2.7Te0.3 is likely to form with the addition of Indium into the matrix [40]. In this case, (Bi0.96In0.04)2Se2.7Te0.3 shows 9 times reduction in electrical resistivity value compared to pristine Bi2Se3. The antisite defect equation is represented by
The electrical resistivity at low-temperature (30–200 K) region and near room temperature region (200–350 K) were attempted to fit by variable range hopping model (VRH) and small polaron hopping (SPH) model [41, 42], and the results are shown in Figs. 7 and 8, respectively. The VRH model is given by
where \({\rho }_{0}\) is the pre-exponential factor, and T0 is the characteristic temperature. It is seen that temperature-dependent electrical resistivity for all studied samples is satisfactorily described by the VRH model in the temperature range 10–60 K (see Fig. 7). The VRH model is valid when the thermal energy is not enough to excite the electrons to hop to their adjacent neighbors. It is more favorable for the electrons to hop further to find a site with a minor potential difference. The characteristic temperature is given by
where N(EF) is the density of state at the Fermi level, kB is the Boltzmann constant, and α is the average localization length, calculated using
It was found to be 0.013 nm−1[43]. Using T0 obtained from the slope in Fig. 7, N(EF) is estimated by employing Eq. 4, and the value for each sample is given in Table 3. A clear distinction among the two types of conduction can be made by the pre-exponential factor for the conduction in localized states. In a semiconductor extrinsic regime at sufficiently low temperatures, the charge transport between the localized state in an impurity band allows few charge carriers with hopping from occupied states below the fermi level. Thus, VRH conduction could play a vital role in the charge of transport phenomenon [44, 45]. On the other hand, the temperature-dependent electrical resistivity data for all studied samples are well fitted to the SPH model in the temperature range 200–350 K, and the result is shown in Fig. 8. The SPH model is given by
where EA is the activation energy. The activation energy of each compound is calculated using the obtained slope from Fig. 8. Thermally activated hopping polarons are responsible for the conduction mechanism in high-temperature region [46]. Activation energies of Bi2Se3, Bi2Se2.7Te0.3, (Bi0.98In0.02)2Se2.7Te0.3, and (Bi0.96In0.04)2Se2.7Te0.3 are found to be 15 meV, 17 meV, 22 meV, and 15 meV at 300 K, respectively. As the impurities are introduced to the lattice, there is an increase in the activation energy of samples due to the addition of extra energy levels. Electrons will settle within the conduction band, which elevates the Fermi level to higher energy as a result of the co-doping in Bi2Se3. This is presumably due to the super-stoichiometry and/or point defects that play a major role in the crystal lattice [47]. The change in the effective mass may be the reason for the unexpected decrease in the activation energy of the In-doped sample with x = 0.04. The activation energy of the presently studied compounds is compared with other reports in Table 4 [48,49,50,51].
3.5 Seebeck coefficient
The temperature-dependent Seebeck coefficient (S) of the (Bi1-xInx)2Se2.7Te0.3 (x = 0.00, 0.02, and 0.04) system is displayed in Fig. 9. The pristine Bi2Se3 sample has the highest Seebeck coefficient value of about 123 μV/K at 350 K and shows p-type semiconductor nature. It is well documented that Selenium is significantly vaporized from the sample during crystal growth, which creates lattice vacancies of VSe. Similarly, Bi cations produce the anionic lattice vacancies to generate antisite defect BiSe. The small crystallites in the crystals are joined by grain boundaries of complex structure, as a result disorders are formed in atomic layers. This will cause a significant change in electronic properties [48, 52, 53]. Hence the carrier scattering effect of grain boundary refinement will produce holes which could be the reason for the p-type nature of the compound Bi2Se3 [54]. The rest of the samples has a negative value of the Seebeck coefficient, indicating the n-type semiconductor nature. This is due to the dopants acting as a donor atom by providing extra electrons. Because of the initial substitution of Tellurium in Se2 sites, Bi-(In)-Se2 will be replaced by Bi-(In)-Te2 bonds. This will lead to the mutual bonding of [-Se-Bi-Te-Bi-Se-]. Therefore, by the continuous substitution of Tellurium, Seebeck coefficient becomes negative. As a result, electrical conductivity increases gradually among the samples [55, 56]. It is observed that there is random variation in the Seebeck coefficient of doped samples, which could be due to the unobvious grain growth of microstructure caused by the carrier scattering effect. The carrier scattering factor is calculated using
where γ is the scattering factor, n is the carrier concentration, and S is the Seebeck coefficient [39]. Moreover, Experimental and theoretical calculation of the carrier scattering factor values are in good matching with each other which are only dependent on carrier concentration and Seebeck coefficient. The value of Seebeck coefficient becomes very negligible compared to the natural logarithm of carrier concentration (n) of each sample. Expectedly, the scattering factors are matching well among theoretical and experimental calculations [57].The Hall measurement was performed using the Van Der Paw method with the input current of 50 mA at 300 K, which confirms the p-type nature of the pristine sample and n-type nature for the doped samples, consistent with the Seebeck coefficient measurements. Theoretical Hall effect calculations can be related to Seebeck coefficients by the formula
where S(T) is the Temperature-dependent Seebeck coefficient, kb is the Boltzmann’s constant, m* is the effective mass of electron, e is the charge of an electron, h is the plank’s constant, n is the carrier concentration, and T is the temperature applied [58, 59]. Theoretical values of mobility were calculated using
where \(\rho\) is the electrical resistivity (\(\Omega m\). Theoretical and experimental carrier concentrations, mobility, and scattering factor values are presented in Table 5. The theoretical and experimental carrier concentration values are in the same range of order 1022/m3 which exhibits that the hall effect and the Seebeck coefficients are in good agreement with each other. There is a deviation in values of theoretical hall mobility from experimental hall mobility among the samples, which is presumably due to some unavoidable constraints of the experiment. The order of experimental and theoretical carrier concentration (n) values is found to be 1022/m3 among all the samples. However, there is a variation in the coefficient of n values which could be due to the anisotropic nature of samples (Bi1-xInx)2Se2.7Te0.3. The probable reason for deviation in Hall measurements may be attributed to rapid drop of resistance of four thermal in the direction of high mobility by collective anisotropic orientation, which is a main challenge to measure the precise values of mobility and carrier concentration simultaneously and can be considered as limiting factor for experimental and theoretical deviations [60, 61]. The localization of electron–electron interaction causes co-occurrence of degenerated bulk thermally activated layers with high experimental mobility value in Bi2Se3 (21.7 × 10−4m2/Vs), whereas (Bi0.96In0.04)2Se2.7Te0.3 has 1/7 ratio of experimental and theoretical Hall mobility which is due to the increase in the doping density with hopping conduction by small polarons, which resulted in a discrepancy of theoretical Hall mobility values. Besides the electrons in the conduction band are observed to be localized, as there is not much contribution to the Hall voltage, but continue to contribute to the electrical conductivity [62]. The theoretical values of Hall mobility are calculated using Eq. 9. The theoretical values of Hall mobility are dependent on values of carrier concentration and electrical resistivity of a sample, whereas the experimental values are directly obtained by Hall effect instrument Keithley meter 6220″. Besides the uneven variation of effective mass has made a significant effect on Hall mobility. The effective mass is calculated using Eq. 8 and is shown in Table 5. The Fermi energy (EF) of each compound is calculated by using
and presented in Table 5.
3.6 Thermal conductivity
Figure 10 illustrates the temperature-dependent thermal conductivity κ(T) of (Bi1-xInx)2Se2.7Te0.3 samples in the temperature range 10–350 K. The pristine Bi2Se3 shows a pronounced phonon peak at around 25 K, which is a common feature for crystalline solids [63, 64]. It is seen that the height of the peak is significantly suppressed with doping at low temperatures, suggesting a substantial enhancement in the phonon scattering. Besides, we found that κ(T) decreases with an increase in Indium content for the (Bi1-xInx)2Se2.7Te0.3 system at high temperatures. These findings are presumably due to the lattice point defects in the crystal caused by the substitution which induces mass and size contrast [65]. As a result, the grain boundary scattering and point defect scattering have a significant effect on thermal transport property at low temperatures. The atomic defects such as vacancies and antisite disorder could also reduce thermal conductivity, as the average wavelength of heat-carrying phonons reduces [66].
The total thermal conductivity for crystalline solids is generally expressed as sum of electronic and lattice contributions. The electronic contribution of heat transport κe can be calculated by the Wiedemann–Franz law
where L0 is the Lorentz number, which is equal to 2.45 × 10–8 WΩK−2, and ρ is the electrical resistivity [67, 68]. The lattice component of thermal conductivity (κL) is obtained by subtracting the electronic thermal conductivity from the total thermal conductivity (κ). The electronic and lattice thermal conductivity graphs are shown in Fig. 11. By the separation of electronic and lattice contributions of the total thermal conductivity, it is revealed that the major contribution to the measured κ originates from the phononic component rather than charge carriers. A rapid increase in the lattice thermal conductivity at low temperatures is seen. With further increase of temperature, the lattice thermal conductivity decreases due to the predominate phonon–phonon scattering (Umklapp process) [69]. (Bi0.96In0.04)2Se2.7Te0.3 exhibits 7.5 times reduction in thermal conductivity value compared to pristine Bi2Se3.
3.7 Power factor (PF) and thermoelectric figure of merit (ZT)
The temperature-dependent power factor is calculated in the temperature range 10–350 K using equation PF = S2/ρ, and is shown in the inset Fig. 12. It is seen that there is an increase in PF as the doping concentration increases. The In-doped samples with x = 0.02, 0.04 show the highest power factor of about 120 μW/mK2 at 350 K, which is about twice that of the pristine sample. Correspondingly, the ZT values are calculated as a function of temperature, and the result is shown in Fig. 12. The highest ZT value of about 0.023 was observed for the In-doped samples with x = 0.02 at 350 K, which is about 2.1 times higher than that of the pristine sample. (Bi0.98In0.02)2Se2.7Te0.3 and (Bi0.96In0.04)2Se2.7Te0.3 show better PF and ZT values, mainly due to the significant reduction in electrical resistivity and thermal conductivity. Hence, the co-doped (Bi0.96In0.04)2Se2.7Te0.3, (Bi0.98In0.02)2Se2.7Te0.3 compounds are potential candidate for Peltier cooling applications.
4 Conclusion
In this work, we report low and near room temperature (10–350 K) thermoelectric properties of the (Bi1-xInx)2Se2.7Te0.3 system. The XRD analysis shows that the crystal structure is hexagonal with space group of \(R\stackrel{-}{3}\) m. The surface morphology shows that there is an increase in porosity and volatilization of Selenium in the sample. The sample doped with Indium (x = 0.04) shows a 9 times reduction in electrical resistivity compared to the pristine sample due to the variation in impurity bands. At low temperature, the thermal conductivity was found to reduce by 7.5 times in the In-doped sample with x = 0.04 in comparison with the pristine sample. It is revealed that the phonon–phonon interaction is predominant over the phonon–electron interaction. The Seebeck coefficient and Hall effect measurements confirm the p-type nature for the pristine and n-type nature for the doped samples. The highest ZT values are found to be 0.023 and 0.022 for the In-doped sample with x = 0.02 and 0.04, respectively, with a PF value of about 120 μW/mK2 at 350 K. From the present study, it is realized that co-doping reduces thermal conductivity and electrical resistivity. Hence co-doping may be an alternative strategy to improve the thermoelectric performance of Bi2Se3, (Bi0.98In0.02)2Se2.7Te0.3, and (Bi0.96In0.04)2Se2.7Te0.3 systems.. By suitable optimization, In-doped Bi2Se2.7Te0.3 can be used for low and near room temperature TE applications.
Change history
03 November 2020
The original version is updated due to request on integrate of funding note.
References
H. Julian Goldsmid, Introduction to Thermoelectricity (Springer, Sydney, 2010)
X. Hu, P. Jood, M. Ohta, M. Kunii, K. Nagase, H. Nishiate, A. Yamamoto, power generation from nanostructured PbTe-based thermoelectrics: comprehensive development from materials to modules. Energy Environ. Sci. 9(2), 517–529 (2016)
J.R. Sootsman, D.Y. Chung, M.G. Kanatzidis, New and old concepts in thermoelectric materials. Angew. Chem. Int. 48(4), 8616–8639 (2009)
K. Shyam Prasad, A. Rao, S. Nagendra Chauhan, R. Bhardwaj, A. Vishwakarma, K. Tyagi, Thermoelectric properties of p-type sb-doped Cu2SnSe3 near room and mid temperature applications. Int. J. Appl. Phys. B 124(2), 98–106 (2018)
A.M. Adam, E.M.M. Ibrahim, L.V. Panina, P. Petkov, Optical and thermoelectric properties of nanocrystalline Bi2(Se1-xTex)3 films. Nanoscale Microscale Thermophys. 22(1), 21–38 (2018)
H.M. Ali, E.M.M. Ibrahim, M.M. Wakkad, M.A.A. Mohamed, Effect of the synthesis conditions on the structural, morphological and optical properties of Bi2Te2.7Se0.3 nanoparticles. Optik 158(1), 199–203 (2018)
K. Kadel, L. Kumari, W.Z. Li, J.Y. Huang, P.P. Provencio, Synthesis and thermoelectric properties of Bi2Se3 nanostructures. Nanoscale Res. Lett. 6(1), 57 (2011)
Q. Lognoné, F. Gascoin, O.I. Lebedev, L. Lutterotti, S. Gascoin, D. Chateigner, Quantitative texture analysis of spark plasma textured n-Bi2Te3. J. Am. Ceram. Soc. 97(7), 2038–2045 (2014)
M. Baitimirova, J. Andzane, G. Petersons, R. Meija, R. Poplausks, M. Romanova, D. Erts, Vapor–solid synthesis and enhanced thermoelectric properties of non-planar bismuth selenide nanoplates on graphene substrate. J. Mater. Sci. 51(17), 8224–8232 (2016)
M. Ramezani, A. Sobhani-Nasab, A. Davoodi, Bismuth selenide nanoparticles: simple synthesis, characterization, and its light harvesting applications in the presence of novel precursor. J. Mater. Sci. 26(7), 5440–5445 (2015)
Y. Min, J.W. Roh, H. Yang, M. Park, S.I. Kim, S. Hwang, S.M. Lee, K.H. Lee, U. Jeong, Surfactant-free scalable synthesis of Bi2Te3 and Bi2Se3 nanoflakes and enhanced thermoelectric properties of their nanocomposites. Adv. Mater. 45(10), 1425–1429 (2013)
S. Gupta, N. Vijayan, A. Krishna, K. Thukral, K.K. Maurya, S. Muthiah, A. Dhar, B. Singh, G. Bhagavannarayana, Enhancement of thermoelectric figure of merit in Bi2Se3 crystals through a necking process. J. Appl. Crystallogr. 48(2), 533–541 (2015)
C. Kulsi, A. Ghosh, A. Mondal, K. Kargupta, S. Ganguly, D. Banerjee, Remarkable photo-catalytic degradation of malachite green by nickel doped Bismuth selenide under visible light irradiation. Appl. Surf. Sci. 392(2), 540–548 (2017)
M. Takashiri, K. Kurita, H. Hagino, S. Tanaka, K. Miyazaki, Enhanced thermoelectric properties of phase-separating bismuth selenium telluride thin films via a two-step method. J. Appl. Phys. 118(6), 065301 (2015)
J. Andzane, L. Britala, E. Kauranens, A. Neciporenko, M. Baitimirova, S. Lara-Avila, S. Kubatkin, M. Bechelany, D. Erts, Effect of graphene substrate type on formation of Bi2 Se3 nanoplates. Sci. Rep. 9(1), 181–188 (2019)
Q. Lognoné, F. Gascoin, Reactivity, stability and thermoelectric properties of n-Bi2Te3 doped with different copper amounts. J. Alloys Compd. 610(3), 1–5 (2014)
Z Ding, SC Huang, D Marcus, RB Kaner. Modification of bismuth telluride for improving thermoelectric properties. Eighteenth International Conference on Thermoelectrics. Proceedings, ICT'99 (Cat. No. 99TH8407), Baltimore, MD, USA. (pp. 721–724). IEEE (1999).
I. García, I. Rey-Stolle, B. Galiana, C. Algora, Analysis of tellurium as n-type dopant in GaInP: doping, diffusion, memory effect and surfactant properties. J. Cryst. Growth. 298(5), 794–799 (2007)
B. Akimov, A.V. Dmitriev, D.R. Khokhlov, L.I. Ryabova, Carrier transport and non-equilibrium phenomena in doped PbTe and related materials. Phys. Status Solidi A 137(1), 9–55 (1993)
K.S. Prasad, A. Rao, R. Bhardwaj, K.K. Johri, C.C. Chang, Y.K. Kuo, Spark plasma sintering technique: an alternative method to enhance ZT values of Sb doped Cu2SnSe3. J. Mater. Sci. 29(15), 13200–13208 (2018)
F. Li, R. Zhai, Y. Wu, Z. Xu, X. Zhao, T. Zhu, Enhanced thermoelectric performance of n-type bismuth-telluride-based alloys via in alloying and hot deformation for mid-temperature power generation. J. Mater. 4(3), 208–214 (2018)
N.J. Choudhari, Y. Raviprakash, B.J. Fernandes, N.K. Udayashankar, Role of soaking time on the phase evolution of Cu2ZnSnS4 polycrystals synthesized using melting route for photovoltaic applications. J. Alloys Compd. 799(3), 314–324 (2019)
E. Lifshin (ed.), X-ray Characterization of Materials (Wiley-VCH Publisher, 1965)
A. Altomare, C. Cuocci, C. Giacovazzo, D. Moliterni, R. Rizzi, N. Corriero, A. Falcicchio, EXPO2013: a kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 46(4), 1231–1235 (2013)
P. Scardi, L.B. Mccusker, R.B. Von Dreele, D.E. Cox, D. Loue, Rietveld refinement guidelines. J. Appl. Crystallogr. 8(1), 134 (1999)
Y.L. Huang, S.P. Chiu, Z.X. Zhu, Z.Q. Li, J.J. Lin, Variable-range-hopping conduction processes in oxygen deficient polycrystalline ZnO films. J. Appl. Phys. 107(6), 063715–63721 (2010)
D. Yu, C. Wang, B.L. Wehrenberg, P. Guyot-Sionnest, Variable range hopping conduction in semiconductor nanocrystal solids. Phy. Rev. Lett. 92(21), 216802 (2005)
L.J. Zhang, B.P. Zhang, Z.H. Ge, C.G. Han, Fabrication and properties of Bi2S3−xSex thermoelectric polycrystals. Solid State Commun. 162(6), 48–52 (2013)
S. Wang, W. Xie, H. Li, X. Tang, Enhanced performances of melt spun Bi2(Te, Se)3 for n-type thermoelectric legs. Intermetallics 19(7), 1024–1031 (2011)
Y. Sun, H. Cheng, S. Gao, Q. Liu, Z. Sun, C. Xiao, C. Wu, S. Wei, Y. Xie, Atomically thick bismuth selenide freestanding single layers achieving enhanced thermoelectric energy harvesting. J. Am. Chem. Soc. 134(50), 20294–20297 (2012)
M. Imura, T. Kamiya, T. Nakanishi, K. Nomura, H. Hosono, Intrinsic carrier mobility in amorphous In–Ga–Zn–O thin-film transistors determined by combined field-effect technique. Appl. Phys. Lett. 96(26), 262105 (2010)
F. Li, R. Zhai, Y. Wu, Z. Xu, X. Zhao, T. Zhu, Enhanced thermoelectric performance of n-type bismuth-telluride-based alloys via In alloying and hot deformation for mid-temperature power generation. J. Materiom. 4(3), 208–214 (2018)
G.S. Hegde, A.N. Prabhu, A. Rao, P.D. Babu, Enhancement of thermoelectric performance of In doped Bi2Te2.7Se0.3 compounds. Phys. B 584(3), 412087–412097 (2020)
S. Byun, J. Cha, C. Zhou, Y.K. Lee, H. Lee, S.H. Park, W.B. Lee, I. Chung, Unusual n-type thermoelectric properties of Bi2Te3 doped with divalent alkali earth metals. J. Solid State Chem. 269(3), 396–400 (2019)
L. Wu, X. Li, S. Wang, T. Zhang, J. Yang, W. Zhang, L. Chen, J. Yang, Resonant level-induced high thermoelectric response in indium-doped GeTe. NPG Asia. Mater. 9(1), 343–347 (2017)
D.K. Bhat, U.S. Shenoy, Enhanced thermoelectric performance of bulk tin telluride: synergistic effect of calcium and indium co-doping. Mater. Today Phys. 4, 8–12 (2018)
U.S. Shenoy, D.K. Bhat, Electronic structure engineering of tin telluride through co-doping of bismuth and indium for high performance thermoelectrics: a synergistic effect leading to a record high room temperature ZT in tin tellurie. J. Mater. Chem. C 7(16), 4817–4821 (2019)
H. Xu, G. Chen, R. Jin, D. Chen, J. Pei, Y. Wang, Electrical transport properties of microwave-synthesized Bi2Se3−xTex nanosheet. Cryst. Eng. Commun. 15(28), 5626–5632 (2013)
E.M. Ibrahim, M.A. Mohamed, H.M. Ali, V.O. Khavrus, S. Hampel, M.M. Wakkad, Optical and transport properties of few quintuple-layers of Bi2-xSbxSe3 nanoflakes synthesized by hydrothermal method. J. Alloys Compd. 804(10), 272–280 (2019)
E.K. Shokr, E.M.M. Ibrahim, A.A. Hakeem, A.M. Adam, Structural, electrical, and thermoelectrical properties of (Bi1−xSbx)2Se3 alloys prepared by a conventional melting technique. J. Exp. Theor. Phys. 116(4), 166–172 (2013)
B.S. Nagaraja, A. Rao, P.D. Babu, G.S. Okram, Structural, electrical, magnetic and thermal properties of Gd1–xSrxMnO3 (0.2≤ x≤ 0.5) manganites. Phys. B 479(11), 10–20 (2015)
S. Prasad, A. Rao, B. Gahtori, S. Bathula, A. Dhar, J.S. Du, Y.K. Kuo, The low and high temperature thermoelectric properties of Sb doped Cu2SnSe3. Mater. Res. 83(7), 160–166 (2016)
G.C. Dannangoda, C. Key, M. Sumets, K.S. Martirosyan, Transition of p-to n-type conductivity in mechanically activated bismuth telluride. J. Electron. Mater. 47(10), 5800–5809 (2018)
D. Yu, C. Wang, B.L. Wehrenberg, P. Guyot-Sionnest, Variable range hopping conduction in semiconductor nanocrystal solids. Phy. Rev. Lett. 92(21), 216802–216808 (2004)
K.H. Kim, S. Lara-Avila, H. Kang, H. He, J. Eklöf, S.J. Hong, M. Park, K. Moth-Poulsen, S. Matsushita, K. Akagi, S. Kubatkin, Author correction: apparent power law scaling of variable range hopping conduction in carbonized polymer nanofibers. Sci. Rep. 10(1), 1–8 (2020)
N. Zarrin, S. Husain, S. Sharma, A. Somvanshi, S. Manzoor, W. Khan, Thermally stimulated small polaron promoted conduction mechanism in Fe-doped La0.7Sm0.3CrO3. J. Phys. Chem. Solid 138, 109281–109281 (2020)
E.M. Ibrahim, A.A. Hakeem, A.M. Adam, E.K. Shokr, Effect of heat treatment on the electrical and thermoelectric properties of Sb doped Bi2Se3. Phys. Scr. 90(4), 045802–045807 (2015)
K.J. John, B. Pradeep, E. Mathai, Electrical properties of bismuth selenide (Bi2Se3) thin films prepared by reactive evaporation. Solid State Commun. 85(10), 879–881 (1993)
D. Nataraj, K. Senthil, S.K. Narayandass, D. Mangalaraj, Conduction studies on bismuth selenide thin films, crystal research and technology. J. Exp. Theor. Phys. 34(7), 34867–34872 (1999)
N.S. Patil, A.M. Sargar, S.R. Mane, P.N. Bhosale, Effect of Sb doping on thermoelectric properties of chemically deposited bismuth selenide films. Mater. Chem. Phys. 15(1), 47–51 (2009)
M. Mitra, C. Kulsi, K. Kargupta, S. Ganguly, D. Banerjee, Composite of polyaniline-bismuth selenide with enhanced thermoelectric performance. J. Appl. Polym. 135(48), 46887–46897 (2018)
K. Rothe, M. Stordeur, H.S. Leipner, Power factor anisotropy of p-type and n-type conductive thermoelectric Bi-Sb-Te thin films. J. Electron Mater. 39(9), 1395–1398 (2010)
K.T. Kim, T.S. Min, S.D. Kim, E.A. Choi, D.W. Kim, S.Y. Choi, Strain-mediated point defects in thermoelectric p-type bismuth telluride polycrystalline. Nano Energy 1(6), 486–493 (2019)
M. Ahmad, K. Agarwal, B.R. Mehta, An anomalously high seebeck coefficient and power factor in ultrathin Bi2Te3 film: spin–orbit interaction. J. Appl. Phys. 128(3), 035108–35114 (2020)
A. Kadhim, A. Hmood, H.A. Hassan, Effect of Se substitution on structural and electrical transport properties of Bi0.4Sb1.6Se3xTe3(1–x) hexagonal rods. J. Electron. Mater. 42(6), 1017–1023 (2013)
M.H. Lachance, E.E. Gardner, Thermoelectric properties of the Bi2Te3- Bi3Se3 isomorphic compound system. Adv. Energy Convers. 1, 133–138 (1961)
Y. Wu, Q. Lou, Y. Qiu, J. Guo, Z.Y. Mei, X. Xu, J. Feng, J. He, Z.H. Ge, Highly enhanced thermoelectric properties of nanostructured Bi2S3 bulk materials via carrier modification and multi-scale phonon scattering. Inorg. Chem. Front. 6(6), 1374–1381 (2019)
T. Kamiya, K. Nomura, H. Hosono, Origin of definite hall voltage and positive slope in mobility-donor density relation in disordered oxide semiconductors. Appl. Phys. Lett. 96(12), 122103–122106 (2010)
O. Bierwagen, R. Pomraenke, S. Eilers, W.T. Masselink, Mobility and carrier density in materials with anisotropic conductivity revealed by van der Pauw measurements. Phys. Rev. B 70(16), 165307–165313 (2004)
X. Yan, B. Poudel, Y. Ma, W.S. Liu, G. Joshi, H. Wang, Y. Lan, D. Wang, G. Chen, Z.F. Ren, Experimental studies on anisotropic thermoelectric properties and structures of n-type Bi2Te2.7Se0.3. Nano Lett. 10(9), 3373–3378 (2010)
S. Prasad, A. Rao, B. Christopher, R. Bhardwaj, N.S. Chauhan, S.A. Malik, N. Van Nong, B.S. Nagaraja, R. Thomas, Tuning the thermoelectric properties by manipulating copper in Cu2SnSe3 system. J. Alloys Compd. 748(5), 273–280 (2018)
X.A. Fan, J.Y. Yang, W. Zhu, H.S. Yun, R.G. Chen, S.Q. Bao, X.K. Duan, Microstructure and thermoelectric properties of n-type Bi2Te2.85Se0.15prepared by mechanical alloying and plasma activated sintering. J. Alloys Compd. 31(2), 256–259 (2006)
Z. Zhou, C. Uher, A. Jewell, T. Caillat, Influence of point-defect scattering on the lattice thermal conductivity of solid solution Co (Sb1–xAsx)3. Phy. Rev. B 71(23), 235209–235217 (2005)
W. Kim, Strategies for engineering phonon transport in thermoelectrics. J. Mater. Chem. C 3(40), 10336–10348 (2015)
T.M. Tritt (ed.), Thermal Conductivity: Theory, Properties, and Applications (Springer Science & Business Media publishers, New York, 2005)
W.H. Shin, J.W. Roh, B. Ryu, H.J. Chang, H.S. Kim, S.W.S. Lee, A. Seo, Enhancing thermoelectric performances of bismuth antimony telluride via synergistic combination of multiscale structuring and band alignment by FeTe2 incorporation. ACS Appl. Mater. Interfaces 10(4), 3689–3698 (2018)
J.W. Roh, K. Hippalgaonkar, J.H. Ham, R. Chen, M.Z. Li, P. Ercius, A. Majumdar, W. Kim, W. Lee, Observation of anisotropy in thermal conductivity of individual single-crystalline bismuth nanowires. ACS Nano 5(4), 3954–3960 (2011)
A. Soni, Z. Yanyuan, Y. Ligen, M.K. Aik, M.S. Dresselhaus, Q. Xiong, Enhanced thermoelectric properties of solution grown Bi2Te3–xSex nanoplatelet composites. Nano Lett. 12(3), 1203–1209 (2012)
R. Thomas, A. Rao, R. Bhardwaj, L.Y. Wang, Y.K. Kuo, Reduction in thermal conductivity and electrical resistivity in Cu2SnSe3/Cu2Se composite thermoelectric system. Mater. Res. Bull. 120(7), 110607–110614 (2019)
Acknowledgements
Ganesh Shridhar Hegde (GSH) would like to acknowledge the Manipal Academy of Higher Education for providing financial support from Dr. T. M. A. doctoral fellowship and would like to thank Dr. Dhananjaya Kekuda , Dr. Mahesha M. G., Mr. Parashuram Salunkhe, and Mr. Prashant Bhat for their help. GSH also would like to show gratitude towards Ms. Mahalaxmi Hegde for her help. The electrical and thermal transport measurements were supported by the Ministry of Science and Technology of Taiwan under Grant Nos. MOST-106-2112-M-312 259-002-MY3 and MOST-108-2112-M- 259-001 (YKK).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hegde, G.S., Prabhu, A.N., Huang, R.Y. et al. Reduction in thermal conductivity and electrical resistivity of indium and tellurium co-doped bismuth selenide thermoelectric system. J Mater Sci: Mater Electron 31, 19511–19525 (2020). https://doi.org/10.1007/s10854-020-04383-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04383-7