Abstract
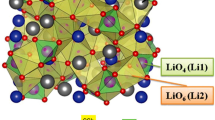
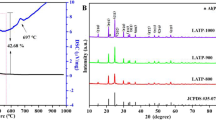
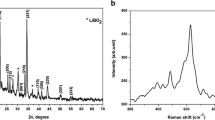
As we all know, solid-state batteries (SSBs) which can effectively inhibit lithium dendrites have disadvantages such as low conductivity and poor cycling performance, and the preparation process of their solid electrolyte is complex and costly, which makes it difficult to meet the requirements of commercialization. Therefore, this paper proposes a strategy to prepare a new solid electrolytes (Li-IL@Al-PILC solid electrolyte) using the nano-2D interlayer structure of Al-pillared clay (Al-PILC) as Li+ channel through an impregnation method. This Li-IL@Al-PILC SSE at 15% Li-IL has many advantages, such as high conductivity (2.13 × 10−3 S/cm at 25 ℃), good thermal stability (up to 450 ℃), extremely wide electrochemical window (up to 5.5 V) and low cost. At the same time, the assembled battery exhibits good cycling performance, with capacities of 120 mAh/g after 1000 cycles at 0.5C for LiFePO4 and good compatibility with the electrode. Due to its good mechanical hardness and nano-2D interlayer interface, Li-IL @ Al-PILC SSE has a certain ability to inhibit the growth of lithium dendrites. Through the above results, it can indicate that Li-IL @ Al-PILC SSE owns a good prospect for lithium metal batteries.













Similar content being viewed by others
References
A. Chandrasekhar, V. Vivekananthan, G. Khandelwal et al., A fully packed water-proof, humidity resistant triboelectric nanogenerator for transmitting Morse code. Nano Energy 60, 850–856 (2019)
A. Chandrasekhar, V. Vivekananthan, S.-J. Kim, A fully packed spheroidal hybrid generator for water wave energy harvesting and self-powered position tracking. Nano Energy 69, 104439 (2020)
A. Chandrasekhar, G. Khandelwal, N.R. Alluri et al., Battery-free electronic smart toys: a step toward the commercialization of sustainable triboelectric nanogenerators. ACS Sustain. Chem. Eng. 6(5), 6110–6116 (2018)
A. Chandrasekhar, V. Vivekananthan, G. Khandelwal et al., A Sustainable human-machine interactive triboelectric nanogenerator towards a smart computer mouse. ACS Sustain. Chem. Eng. 7(7), 7177–7182 (2019)
V. Vivekananthan, A. Chandrasekhar, N.R. Alluri et al., Fe2O3 magnetic particles derived triboelectric-electromagnetic hybrid generator for zero-power consuming seismic detection. Nano Energy 64, 103926 (2019)
M. Li, J. Lu, Z. Chen et al., 30 Years of lithium-ion batteries. Adv. Mater. 30(33), 1800561 (2018)
J.-M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414(6861), 359–367 (2001)
A. Manthiram, X. Yu, S. Wang, Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2(3), 16103 (2017)
F. Lei, S. Wei, S. Li et al., Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater. 8(11), 1702657 (2018)
D. Lin, Y. Liu, Y. Cui, Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12(3), 194 (2017)
J. Lu, Z. Chen, F. Pan et al., High-performance anode materials for rechargeable lithium-ion batteries. Electrochem. Energy Rev. 1(1), 35–53 (2018)
M.D. Tikekar, S. Choudhury, Z. Tu et al., Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1(9), 16114 (2016)
A. Sharafi, S. Yu, M. Naguib et al., Impact of air exposure and surface chemistry on Li-Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 5(26), 13475–13487 (2017)
C. Deviannapoorani, L. Dhivya, S. Ramakumar et al., Lithium ion transport properties of high conductive tellurium substituted Li7La3Zr2O12 cubic lithium garnets. J. Power Sources 240, 18–25 (2013)
Y. Inaguma, C. Liquan, M. Itoh et al., High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 86(10), 689–693 (1993)
V. Thangadurai, H. Kaack, W.J.F. Weppner, Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 86(3), 437–440 (2003)
L. Li, S. Liu, A. Manthiram, Co3O4 nanocrystals coupled with O- and N-doped carbon nanoweb as a synergistic catalyst for hybrid Li-air batteries. Nano Energy 12, 852–860 (2015)
J. Mindemark, M.J. Lacey, T. Bowden et al., Beyond PEO—alternative host materials for Li + -conducting solid polymer electrolytes. Prog. Polym. Sci. 81, S0079670017301946 (2018)
Y.-J. Heo, H.I. Lee, J.W. Lee et al., Optimization of the pore structure of PAN-based carbon fibers for enhanced supercapacitor performances via electrospinning. Compos. B 161, 10–17 (2019)
Y. Gao, Z. Yan, J.L. Gray et al., Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18(4), 384 (2019)
Y. Tian, F. Ding, H. Zhong et al., Li6.75 La3Zr1.75Ta0.25O12 @amorphous Li3OCl composite electrolyte for solid state lithium-metal batteries. Energy Storage Mater. 14, S2405829718300965 (2018)
Y. Wang, E. Sahadeo, G. Rubloff et al., High-capacity lithium sulfur battery and beyond: a review of metal anode protection layers and perspective of solid-state electrolytes. J. Mater. Sci. 54(5), 3671–3693 (2019)
M.P. Singh, R.K. Singh, S. Chandra, Ionic liquids confined in porous matrices: physicochemical properties and applications. Prog. Mater Sci. 64(10), 73–120 (2014)
A. Fernicola, F. Croce, B. Scrosati et al., LiTFSI-BEPyTFSI as an improved ionic liquid electrolyte for rechargeable lithium batteries. J. Power Sources 174(1), 342–348 (2007)
F.J. Wishart, Energy applications of ionic liquids. Energy Environ. Sci. 2(9), 956 (2009)
J.L. Bideau, L. Viau, A. Vioux, Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 40(2), 907–925 (2011)
N. Chen, Y. Dai, Y. Xing et al., Biomimetic ant-nest ionogel electrolyte boosts the performance of dendrite-free lithium batteries. Energy Environ. Sci. 10(7), 1660–1667 (2017)
R. Ameloot, M. Aubrey, B.M. Wiers et al., Ionic conductivity in the metal-organic framework UiO-66 by dehydration and insertion of lithium tert-butoxide. Chemistry A 19(18), 5533–5536 (2013)
B.M. Wiers, M.-L. Foo, N.P. Balsara et al., A solid lithium electrolyte via addition of lithium isopropoxide to a metal-organic framework with open metal sites. J. Am. Chem. Soc. 133(37), 14522–14525 (2011)
K. Fujie, R. Ikeda, K. Otsubo et al., Lithium ion diffusion in a metal-organic framework mediated by an ionic liquid. Chem. Mater. 27(21), 7355–7361 (2015)
A.K. Tripathi, Y.L. Verma, V.K. Singh et al., Quasi solid-state electrolytes based on ionic liquid (IL) and ordered mesoporous matrix MCM-41 for supercapacitor application. J. Solid State Electrochem. 21(11), 1–7 (2017)
H. Chen, H. Tu, C. Hu et al., Cationic covalent organic framework nanosheets for fast Li-ion conduction. J. Am. Chem. Soc. 140(3), 896–899 (2018)
L. Han, Z. Wang, D. Kong et al., An ordered mesoporous silica framework based electrolyte with nanowetted interfaces for solid-state lithium batteries. J. Mater. Chem. A 6(43), 21280–21286 (2018)
Z. Wang, R. Tan, H. Wang et al., A metal-organic-framework-based electrolyte with nanowetted interfaces for high-energy-density solid-state lithium battery. Adv. Mater. 30(2), 1704436 (2017)
W.J. Roth, B. Gil, W. Makowski et al., Layer like porous materials with hierarchical structure. Chem. Soc. Rev. 45(12), 3400 (2015)
Y. Leng, Q. Li, Q. Tian et al., (Ce-Al)-oxide pillared bentonite: a high affinity sorbent for plutonium. J. Hazard. Mater. 352, 121 (2018)
J. Heard, E. Cejka, O. Maksym et al., 2D oxide nanomaterials to address the energy transition and catalysis. Adv. Mater. 31(3), 1801712 (2018)
A.M. Dunaev, V.B. Motalov, L.S. Kudin et al., Molecular and ionic composition of saturated vapor over EMImNTf 2 ionic liquid. J. Mol. Liq. 219, 599–601 (2016)
E. Bolimowska, F. Castiglione, J. Devemy et al., Investigation of Li+ cation coordination and transportation, by molecular modeling and NMR studies, in a LiNTf2-doped ionic liquid-vinylene carbonate mixture. J. Phys. Chem. B 122(36), 8560–8569 (2018)
Z. Mensinger, W. Wang, D. Keszler et al., Oligomeric group 13 hydroxide compounds—a rare but varied class of molecules. Chem. Soc. Rev. 41, 1019–1030 (2011)
K. Li, J. Lei, G. Yuan et al., Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics. Chem. Eng. J. 317, 800–809 (2017)
P.F. Low, I. Ravina, J.L. White, Changes in b dimension of Na-Mont-morillonite with interlayer swelling. Nature 226(5244), 445–446 (1970)
K. Bahranowski, A. Kielski, J. Podobi et al., Physico chemical characterization and catalytic properties of copper-doped alumina-pillared montmorillonites. Clays Clay Miner. 46(1), 98–102 (1998)
K. Bahranowski, R. Dula, M. Łabanowska et al., ESR study of Cu centers supported on Al-, Ti-, and Zr-pillared montmorillonite clays. Appl. Spectrosc. 50(11), 1439–1445 (1996)
A.M.D. Andrés, J. Merino, J.C. Galván et al., Synthesis of pillared clays assisted by microwaves. Mater. Res. Bull. 34(4), 641–651 (1999)
W.H. Casey, Large aqueous aluminum hydroxide molecules. Chem. Rev. 106(1), 1–16 (2006)
C. Pesquera, C. Blanco, F. González, Montmorillonites with mixed aluminum-lanthanide oxide pillars, in Materials Syntheses: A Practical Guide, ed. by U. Schubert, N. Hüsing, R. Laine (Springer-Verlag, Wien, New York, 2008), pp. 59–63
S. Choudhury, R. Mangal, A. Agrawal et al., A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 6, 10101 (2015)
A.K. Tripathi, R.K. Singh, Development of ionic liquid and lithium salt immobilized MCM-41 quasi solid-liquid electrolytes for lithium batteries. J. Energy Storage 15, 283–291 (2018)
A. Wang, S. Kadam, L. Hong et al., Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Comput. Mater. 4(1), 15 (2018)
A. Manthiram, X. Yu, S. Wang, Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2(4), 16103 (2017)
S. Kim, H. Oguchi, N. Toyama et al., A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries. Nat. Commun. 10(1), 1–9 (2019)
T. Matsuo, Y. Gambe, Y. Sun et al., Bipolar stacked quasi-all-solid-state lithium secondary batteries with output cell potentials of over 6 V. Sci. Rep. 4, 6084 (2014)
Z. Huang, R.-A. Tong, J. Zhang et al., Blending Poly(ethylene oxide) and Li6.4La3Zr1.4Ta0.6O12 by Haake Rheomixer without any solvent: a low-cost manufacture method for mass production of composite polymer electrolyte. J. Power Sources 451, 227797 (2020)
D. Shao, L. Yang, K. Luo et al., Preparation and performances of the modified gel composite electrolyte for application of quasi-solid-state lithium sulfur battery. Chem. Eng. J. 389, 124300 (2020)
L. Chen, W. Li, L.Z. Fan et al., Intercalated electrolyte with high transference number for dendrite-free solid-state lithium batteries. Adv. Funct. Mater. 29, 1901047 (2019)
S. Kim, H. Oguchi, N. Toyama et al., A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries. Nat. Commun. 10, 1–9 (2019)
Y. Tian, F. Ding, H. Zhong et al., Li6.75La3Zr1.75Ta0.25O12@amorphous Li3OCl composite electrolyte for solid state lithium-metal batteries. Energy Storage Mater. 14, 49–57 (2018)
S.-J. Choi, S.-H. Choi, A.D. Bui et al., LiI-doped sulfide solid electrolyte: enabling a high-capacity slurry-cast electrode by low-temperature post-sintering for practical all-solid-state lithium batteries. ACS Appl. Mater. Interfaces. 10(37), 31404–31412 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mao, H., Ding, Z. Electrolytes based on nano-2D interlayer structure of Al-pillared clays for solid-state lithium battery. J Mater Sci: Mater Electron 31, 13874–13888 (2020). https://doi.org/10.1007/s10854-020-03947-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03947-x