Abstract
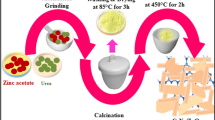
Template/surfactant-free ZnO nanoparticles were synthesized by hydrothermal process at 120 °C, 150 °C, 180 °C and 210 °C for 3-h duration and were annealed at 400 °C for 3 h. Since the ZnO nanoparticles synthesized at 150 °C for 3 h and annealed at 400 °C for 3 h showed improved photocatalytic activities, the ZnO nanoparticles were further synthesized at 150 °C for 6-h, 9-h, and 12-h durations and were annealed at 400 °C for 3 h. All the synthesized ZnO nanoparticles were characterized for their structural, optical, and morphological properties. X-ray diffraction analysis confirmed that the ZnO nanoparticles belong to the hexagonal wurtzite system. Transmission electron microscopy and high-resolution transmission electron microscopy analyses revealed that the hydrothermally synthesized ZnO nanoparticles at 150 °C for 3 h and annealed at 400 °C for 3 h acquired spherical and hexagonal morphologies with size ~ 50 nm. Variation in the hydrothermal temperatures and time durations with annealing at 400 °C for 3 h acquired nearly hexagonal, spheroidal, and hexagonal morphology of the ZnO nanoparticles. Optical band gap of synthesized ZnO nanoparticles was slightly influenced by the different synthesis parameters. The photocurrent measurements revealed that the ZnO nanoparticles synthesized at 150 °C for 3 h and annealed at 400 °C for 3 h possess relatively enhanced of photocurrent of about 3.58 μA than that of the other synthesized samples. The ZnO nanoparticles synthesized at 150 °C for 3 h and annealed at 400 °C for 3 h showed ~ 78% and 36% of photodegradation efficiency against Rhodamine B dye and 4-chlorophenol, respectively, in a time period of 60 min. The sonophotocatalytic activity process enhanced degradation efficiency to 99% in 60 min against Rhodamine B and 80% in 60 min against 4-chlorophenol.


















Similar content being viewed by others
References
C. Allegre, M. Maisseu, F. Charbit, P. Moulin, Coagulation–flocculation–decantation of dye house effluents: concentrated effluents. J. Hazard. Mater. B 116, 57–64 (2004). https://doi.org/10.1016/j.jhazmat.2004.07.005
V. Golob, A. Vinder, M. Simonic, Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dyes Pigments 67, 93–97 (2005). https://doi.org/10.1016/j.dyepig.2004.11.003
A. Alinsafi, M. Khemis, M.N. Pons, J.P. Leclerc, A. Yacoubi, A. Benhammou, A. Nejmeddine, Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Proc. 44, 461–470 (2005). https://doi.org/10.1016/j.cep.2004.06.010
S. Papic, N. Koprivanac, A. Loncaric Bozic, A. Metes, Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigments 62, 291–298 (2004)
A. Bhatnagar, M. Sillanp, Removal of natural organic matter (NOM) and its constituents from water by adsorption. Chemosphere 166, 497–510 (2017). https://doi.org/10.1016/j.chemosphere.2016.09.098
X. Liang, Y. Lu, Z. Li, C. Yang, C. Niu, X. Su, Bentonite/carbon composite as highly recyclable adsorbents for alkaline wastewater treatment and organic dye removal. Microporous Mesoporous Mater. 241, 107–114 (2017). https://doi.org/10.1016/j.micromeso.2016.12.016
T. Robinson, G. McMullan, R. Marchant, P. Nigam, Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technol. 77, 247–255 (2001). https://doi.org/10.1016/S0960-8524(00)00080-8
T.A. Saleh, A. Sari, M. Tuzen, Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 307, 230–238 (2017). https://doi.org/10.1016/j.cej.2016.08.070
F. Fan, Y. Feng, P. Tang, D. Li, Facile synthesis and photocatalytic performance of ZnO nanoparticles self-assembled spherical aggregates. Mater. Lett. 158, 290–294 (2015). https://doi.org/10.1016/j.matlet.2015.05.109
J. Xu, Y. Cui, Y. Han, M. Hao, X. Zhang, ZnO-graphene composites with high photocatalytic activities under visible light. RSC Adv. 6, 96778–96784 (2016). https://doi.org/10.1039/C6RA19622E
N.B. Bokhale, S.D. Bomble, R.R. Dalbhanjan, D.D. Mahale, S.P. Hinge, B.S. Banerjee, A.V. Mohod, P.R. Gogate, Sonocatalytic and sonophotocatalytic degradation of rhodamine 6G containing wastewaters. Ultrason. Sonochem. 21, 1797–1804 (2014). https://doi.org/10.1016/j.ultsonch.2014.03.022
K. Tanaka, K. Padermpole, T. Hisanaga, Photocatalytic degradation of commercial azo dyes. Water Res. 34, 327–333 (2000). https://doi.org/10.1016/S0043-1354(99)00093-7
H. Tong, S.X. Ouyang, Y.P. Bi, N. Umezawa, M. Oshikiri, J.H. Ye, Nano-photocatalytic materials: possibilities and challenges. Adv. Mater. 24, 229–251 (2012). https://doi.org/10.1002/adma.201102752
A.B. Djurisic, Y.H. Leung, A.M.C. Ng, Strategies for improving the efficiency of semiconductor metal oxide photocatalysis. Mater. Horiz. 1, 400–410 (2014). https://doi.org/10.1039/C4MH00031E
F. Seker, K. Meeker, T.F. Kuech, A.B. Ellis, Surface chemistry of prototypical bulk II-VI and III-V semiconductors and implications for chemical sensing. Chem. Rev. 100, 2505–2536 (2000). https://doi.org/10.1021/cr980093r
A. Mclaren, T.V. Solis, G. Li, S.C. Tsang, Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc. 131, 12540–12541 (2009). https://doi.org/10.1021/ja9052703
G.K. Mor, K. Shankar, M. Paulose, O.K. Varghese, C.A. Grimes, Enhanced photocleavage of water using titania nanotube arrays. Nano Lett. 5, 191–195 (2005)
X. Han, Q. Kuang, M. Jin, Z. Xie, L. Zheng, Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 131, 3152–3153 (2009)
Z. Zhang, M.F. Hossain, T. Takahashi, Fabrication of shape-controlled α-Fe2O3 nanostructures by sonoelectrochemical anodization for visible light photocatalytic application. Mater. Lett. 64, 435–438 (2010). https://doi.org/10.1016/j.matlet.2009.10.071
P. Karthik, R. Vinoth, P. Selvam, E. Balaraman, M. Navaneethan, Y. Hayakawa, B. Neppolian, Visible-Light active catechol-metal oxide carbonaceous polymeric material for enhanced photocatalytic activity. J. Mater. Chem. A 5, 384–396 (2017). https://doi.org/10.1039/C6TA07685H
Q.I. Rahman, Musheer Ahmad, Sunil Kumar Misra, Minaxi Lohani, Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 91, 170–174 (2013). https://doi.org/10.1016/j.matlet.2012.09.044
S. Sakthivel, B. Neppolian, M.V. Shankar, B. Arabindoo, M. Palanichamy, V. Murugesan, Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 77, 65–82 (2003). https://doi.org/10.1016/S0927-0248(02)00255-6
C.S. Chen, X.D. Xie, T.G. Liu, L.W. Lin, J.C. Kuang, X.L. Xie, L.J. Lu, S.Y. Cao, Multi-walled carbon nanotubes supported Cu-doped ZnO nanoparticles and their optical property. J. Nanoparticle Res. 14, 817–825 (2012)
X. Wang, L. Yin, G. Liu, L. Wang, R. Saito, G.Q. Lu, H.-M. Cheng, Polar interface-induced improvement in high photocatalytic hydrogen evolution over ZnO-CdS heterostructures. Energy Environ. Sci. 4, 3976–3979 (2011)
M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemann, Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995). https://doi.org/10.1021/cr00033a004
E.S. Jang, J.-H. Won, S.-J. Hwang, J.-H. Choy, Fine tuning of the face orientation of ZnO crystals to optimize their photocatalytic activity. Adv. Mater. 18, 3309–3312 (2006)
J. Liu, X. Chen, W. Wang, Y. Liu, Q. Huanga, Z. Guo, Self-assembly of [10-10] grown ZnO nanowhiskers with exposed reactive (0001) facets on hollow spheres and their enhanced gas sensitivity. CrystEngComm 13, 3425–3431 (2011). https://doi.org/10.1039/C0CE00821D
Y. Yuan, G.F. Huang, W.Y. Hu, D.N. Xiong, W.Q. Huang, Tunable synthesis of various ZnO architectural structures with enhanced photocatalytic activities. Mater. Lett. 175, 68–71 (2016). https://doi.org/10.1016/j.matlet.2016.03.138
X.-G. Han, H.-Z. He, Q. Kuang, X. Zhou, X.-H. Zhang, T. Xu, Z.-X. Xie, L.-S. Zheng, Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J. Phys. Chem. C 113, 584–589 (2009)
J. Xu, Z. Xue, N. Qin, Z. Cheng, Q. Xiang, The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sensor Actuat. B-Chem. 242, 148–157 (2017). https://doi.org/10.1016/j.snb.2016.09.193
J.H. Zeng, B.B. Jin, Y.F. Wang, Facet enhanced photocatalytic effect with uniform single-crystalline zinc oxide nanodisks. Chem. Phys. Lett. 472, 90–95 (2009). https://doi.org/10.1016/j.cplett.2009.02.082
D. Raoufi, Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 50, 932–937 (2013). https://doi.org/10.1016/j.renene.2012.08.076
L. Zhang, L. Yin, C. Wang, N. Lun, Y. Qi, Sol-gel growth of hexagonal faceted ZnO prism quantum dots with polar surfaces for enhanced photocatalytic activity. Appl. Mater. Interfaces 2, 1769–1773 (2010). https://doi.org/10.1021/am100274d
H. Lu, S. Wang, L. Zhao, J. Li, B. Donga, Z. Xu, Hierarchical ZnO microarchitectures assembled by ultrathin nanosheets: hydrothermal synthesis and enhanced photocatalytic activity. J. Mater. Chem. 21, 4228–4234 (2011). https://doi.org/10.1039/C0JM03390A
J. Das, D. Khushalani, Nonhydrolytic Route for Synthesis of ZnO and its use as a recyclable photocatalyst. J. Phys. Chem. C 114, 2544–2550 (2010). https://doi.org/10.1021/jp910773v
R.S. Yadav, P. Mishra, A.C. Pandey, Growth mechanism and optical property of ZnO nanoparticles synthesized by sonochemical method. Ultrason. Sonochem. 15, 863–868 (2008)
S.B. Babar, N.L. Gavade, J. Park, K.M. Garadkar, V.M. Bhuse, Effect of leavening agent on structural and photocatalytic properties of ZnO nanorods. J. Mater. Sci.: Mater. Electron. 28, 8372–8381 (2017). https://doi.org/10.1007/s10854-017-6554-x
S.M. Mousavi, A.R. Mahjoub, R. Abazari, Green synthesis of ZnO hollow sphere nanostructures by facile route at room temperature with efficient photocatalytic dye degradation. RSC Adv. 5, 107378–107388 (2015). https://doi.org/10.1039/C5RA19507A
M. Cao, F. Wang, J. Zhu, X. Zhang, Y. Qin, L. Wang, Shape-controlled synthesis of flower-like ZnO microstructures and their enhanced photocatalytic properties. Mater. Lett. 192, 1–4 (2017). https://doi.org/10.1016/j.matlet.2017.01.051
R. Khan, M.S. Hassan, L.W. Jang, J.H. Yun, H.K. Ahn, M.S. Khil, I.H. Lee, Low temperature synthesis of ZnO quantum dots for photocatalytic degradation of methyl orange dye under UV irradiation. Ceram. Int. 40, 14827–14831 (2014). https://doi.org/10.1016/j.ceramint.2014.06.076
B. Neppolian, A. Doronila, M. Ashokkumar, Sonochemical oxidation of arsenic(III) to arsenic(V) using potassium peroxydisulfate as an oxidizing agent. Water. Res. 44, 3687–3695 (2010). https://doi.org/10.1016/j.watres.2010.04.003
R. Vinoth, P. Karthik, K. Devan, B. Neppolian, M. Ashokkumar, TiO2-NiO p-n nanocomposite with enhanced sonophotocatalytic activity under diffused sunlight. Ultrason. Sonochem. 35, 655–663 (2017). https://doi.org/10.1016/j.ultsonch.2016.03.005
S. GaneshBabu, R. Vinoth, B. Neppolian, D. Dionysiou, M. Ashokkumar, Diffused sunlight driven highly synergistic pathway for complete mineralization of organic contaminants using reduced graphene oxide supported photocatalyst. J. Hazard. Mater. 291, 83–92 (2015)
B. Neppolian, A. Bruno, C.L. Bianchi, M. Ashokkumar, Graphene oxide based Pt–TiO2 photocatalyst: ultrasound assisted synthesis, characterization and catalytic efficiency. Ultrason. Sonochem. 19, 9–15 (2012)
Y. He, F. Grieser, M. Ashokkumar, The mechanism of sonophotocatalytic degradation of methyl orange and its products in aqueous solutions. Ultrason. Sonochem. 18, 974–980 (2011). https://doi.org/10.1016/j.ultsonch.2011.03.017
N. Kumaresan, K. Ramamurthi, R. RameshBabu, K. Sethuraman, S. MoorthyBabu, Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl. Surf. Sci. 418, 138–146 (2017)
B.D. Cullity (1978) Elements of X-Ray Diffraction, 2nd edn. Addison-Wesley, Reading
R. Romero, D. Leinen, E.A. Dalchiele, J.R. Ramos-Barrado, F. Martín, The effects of zinc acetate and zinc chloride precursors on the preferred crystalline orientation of ZnO and Al-doped ZnO thin films obtained by spray pyrolysis. Thin Solid Films 515, 1942–1949 (2006). https://doi.org/10.1016/j.tsf.2006.07.152
W.-K. Jo, J.Y. Lee, N.C.S. Selvam, Synthesis of MoS2 nanosheets loaded ZnO–g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 289, 306–318 (2016). https://doi.org/10.1016/j.cej.2015.12.080
R. Atchudan, T.N.J.I. Edison, S. Perumal, D. Karthikeyan, Y.R. Lee, Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J. Photochem. Photobiol. B 162, 500–510 (2016)
S. Kumar, A. Baruah, S. Tonda, B. Kumar, V. Shanker, B. Sreedhar, Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core–shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale 6, 4830–4842 (2014). https://doi.org/10.1039/C3NR05271K
B. Archana, K. Manjunath, G. Nagaraju, K.B. Chandra Sekhar, N. Kottam, Enhanced photocatalytic hydrogen generation and photostability of ZnO nanoparticles obtained via green synthesis. Int. J. Hydrogen Energy 42, 5125–5131 (2017)
J. Wang, Z. Wang, B. Huang, Y. Ma, Y. Liu, X. Qin, X. Zhang, Y. Dai, Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 4, 4024–4030 (2012). https://doi.org/10.1021/am300835p
D. Chen, Z. Wang, T. Ren, H. Ding, W. Yao, R. Zong, Y. Zhu, Influence of defects on the photocatalytic activity of ZnO. J. Phys. Chem. C 118, 15300–15307 (2014). https://doi.org/10.1021/jp5033349
P. Kubelka, New contributions to the optics of intensely light-scattering materials. Part I. J. Opt. Soc. Am. 38, 448–457 (1948). https://doi.org/10.1364/JOSA.38.000448
Y.C. Kong, D.P. Yu, B. Zhang, W. Fang, S.Q. Feng, Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 78, 407–409 (2001). https://doi.org/10.1063/1.1342050
G. Patrinoiu, M. Tudose, J.M. Calderon-Moreno, R. Birjega, P. Budrugeac, R. Ene, O. Carp, A green chemical approach to the synthesis of photoluminescent ZnO hollow spheres with enhanced photocatalytic properties. J. Solid State Chem. 186, 17–22 (2012). https://doi.org/10.1016/j.jssc.2011.11.024
K. Venheusden, W.L. Warren, C.H. Seager, D.R. Tallant, J.A. Voigt, Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 79, 7983–7990 (1996). https://doi.org/10.1063/1.362349
K. Byrappa, M. Yoshimura, Handbook of Hydrothermal Technology (Noyes Publications, Park Ridge, 2001)
H. Hayashi, Y. Hakuta, Hydrothermal synthesis of metal oxide nanoparticles in supercritical water. Materials 3, 3794–3817 (2010). https://doi.org/10.3390/ma3073794
S.G. Anju, S. Yesodharan, E.P. Yesodharan, Zinc oxide mediated sonophotocatalytic degradation of phenol in water. Chem. Eng. J. 189–190, 84–93 (2012). https://doi.org/10.1016/j.cej.2012.02.032
I.M. Khokhawala, P.R. Gogate, Degradation of phenol using a combination of ultrasonic and UV irradiations at pilot scale operation. Ultrason. Sonochem. 17, 833–838 (2010). https://doi.org/10.1016/j.ultsonch.2010.02.012
C. Lops, A. Ancona, K.D. Cesare, B. Dumontel, N. Garino, G. Canavese, S. Hérnandez, V. Cauda, Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B 243, 629–640 (2019). https://doi.org/10.1016/j.apcatb.2018.10.078
Acknowledgements
One of the authors (N. K) thanks SRM Institute of Science and Technology, Chennai for the award of SRM Institute of Science and Technology Junior Research Fellowship to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumaresan, N., Maria Angelin Sinthiya, M., Praveen Kumar, M. et al. Investigation on the photocatalytic and sonophotocatalytic activities of {002} facets of ZnO nanoparticles synthesized through template/surfactant-free hydrothermal method at different temperatures and time durations. J Mater Sci: Mater Electron 31, 13817–13837 (2020). https://doi.org/10.1007/s10854-020-03942-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03942-2